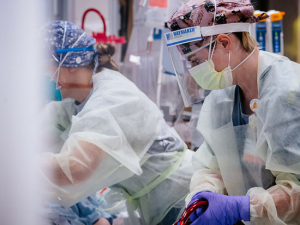
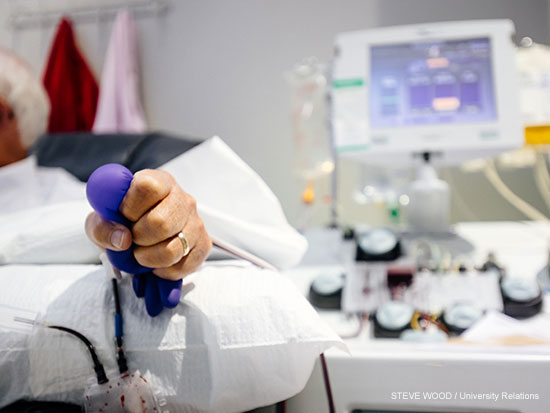
 Convalescent plasma therapy transfuses strong antibodies from recovered COVID-19 patients (such as the donor shown above) into patients fighting the disease to help those sick patients combat the virus.Convalescent plasma therapy — transfusing strong antibodies from recovered COVID-19 patients into patients fighting the disease — has been used to treat patients at UAB Hospital since the beginning of May as part of an expanded access clinical trial in collaboration with the Mayo Clinic. Three months later, with cases in Alabama continuing to rise, UAB has opened two new studies that are expanding the therapy to outpatients and even people who have been exposed to someone with COVID-19 but don’t yet have symptoms themselves.
Convalescent plasma therapy transfuses strong antibodies from recovered COVID-19 patients (such as the donor shown above) into patients fighting the disease to help those sick patients combat the virus.Convalescent plasma therapy — transfusing strong antibodies from recovered COVID-19 patients into patients fighting the disease — has been used to treat patients at UAB Hospital since the beginning of May as part of an expanded access clinical trial in collaboration with the Mayo Clinic. Three months later, with cases in Alabama continuing to rise, UAB has opened two new studies that are expanding the therapy to outpatients and even people who have been exposed to someone with COVID-19 but don’t yet have symptoms themselves.
UAB has treated more than 60 patients with convalescent plasma therapy, “from people as young as 5 weeks to as old as the mid to late 70s,” said Professor Sonya Heath, M.D., of the Division of Infectious Diseases. Across the country, more than 77,000 patients have received the therapy.
Controversy recently arose during the Food and Drug Administration’s deliberations on granting an Emergency Use Authorization (EUA) for convalescent plasma, which the agency did Aug. 23, 2020. Many scientists cautioned that more research is needed. The president of the Infectious Diseases Society of America, Thomas File Jr., M.D., noted in a statement that “while the data to date show some positive signals that convalescent plasma can be helpful in treating individuals with COVID-19, especially if given early in the trajectory of the disease, we lack the randomized, controlled-trial data we need to better understand its utility in COVID-19 treatment.”
Existing studies have not had a control group, making it impossible to statistically demonstrate that patients who received convalescent plasma treatment fared better than those who did not. Furthermore, convalescent plasma has only been used in hospitalized patients, Heath said, and studies to date suggest that earlier use in the course of disease may be more beneficial.
The two new studies are enrolling non-hospitalized patients at UAB to address these knowledge gaps, Heath said. So what’s the latest on convalescent plasma, who is eligible for the new trials and who can donate? Heath answered our questions.
What does the research say on the effectiveness of convalescent plasma?
“First, plasma has been used as a treatment for people who are infected with various diseases since the 1800s,” Heath said. “As far as convalescent plasma therapy for COVID-19, the Mayo Clinic published a report on more than 25,000 people who have received convalescent plasma in hospitals, and it was very safe.” Because the therapy, at UAB and other locations participating in Mayo’s Expanded Access Program, is offered to all who need it, “there is no control group to compare it to,” Heath said. That means there is no way to get a rigorous assessment of the effectiveness of the treatment. If a patient recovered after receiving the treatment, it is not clear if the convalescent plasma was responsible or if the patient would have recovered regardless. Answering the effectiveness question is a key aim of two new randomized controlled clinical trials, led by researchers at Johns Hopkins University. Heath is principal investigator for the trials at UAB.
In terms of donations, “we know that people develop variable antibody responses — not all are created equally,” Heath said. The American Red Cross and LifeSouth have both instituted programs to evaluate the strength of a donor’s antibodies at the time they donate plasma and before it is infused, she said. “That is a significant advancement. We still have unanswered questions about how much antibody is needed, but the fact that the American Red Cross and LifeSouth are doing this screening is telling us that the quality of the antibody we are transfusing is likely getting progressively better with time.”
This is especially beneficial considering that “we also now know that with time people’s antibody responses actually wane,” Heath said. “Not everyone who develops COVID-19 develops antibodies and even in the people who do develop antibodies, those antibodies do not persist long-term. There is growing evidence across the country and with our group at UAB that over a series of several months that people have decreased levels of antibodies.”
What are the two new trials that UAB is participating in?
"Unlike the Mayo Clinic Expanded Access Program trial — which is for patients in the hospital, and will continue — these are trials of the treatment of COVID-positive patients in an outpatient setting, as well as giving plasma to people who have had a high-risk exposure to someone with COVID — for example, someone in their household — in an attempt to prevent them from developing the disease,” Heath said.
“These will hopefully get us closer to understanding the benefits of convalescent plasma therapy to treat COVID-19,” Heath added. “We believe that the earlier you get that treatment, the more effective that treatment will be, so these new trials will help to test that.”
To learn more about this and other clinical trials at UAB, call 205-996-4099 to determine if you are eligible to enroll. |
These trials, unlike the Mayo Clinic trial, will use placebos. Half of participants will be randomized to receive plasma with very high levels of antibodies against COVID-19. The other half will receive plasma banked before the epidemic, which does not have any anti-COVID-19 antibodies. “The study is to evaluate whether plasma with very high levels of antibodies is beneficial,” Heath said. “The endpoint of the trial is hospitalization — the goal is to prevent people with illness from progressing and needing hospitalization,” she explained.
While some participants will not receive active antibodies, it is important to note that this does not mean they will be foregoing any alternative forms of treatment. “All we have now to treat people in the outpatient settings is tylenol, ibuprofen and cold medicines,” Heath said. Remdesivir and steroids, two treatments that have been shown to be effective against COVID-19, are used for hospitalized patients, she said.
“This is an opportunity for people who have infection to not only get a treatment but contribute to advancing understanding of treatments in the outpatient setting,” Heath said.
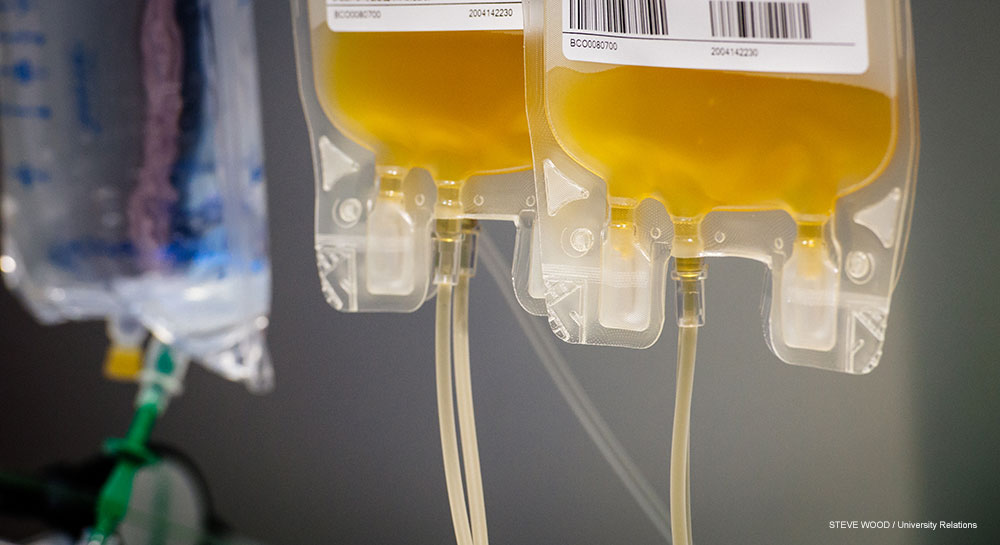 “These [trials] will hopefully get us closer to understanding the benefits of convalescent plasma therapy to treat COVID-19," said Sonya Heath, M.D., principal investigator for the new studies at UAB. "We believe that the earlier you get that treatment, the more effective that treatment will be, so these new trials will help to test that.”
“These [trials] will hopefully get us closer to understanding the benefits of convalescent plasma therapy to treat COVID-19," said Sonya Heath, M.D., principal investigator for the new studies at UAB. "We believe that the earlier you get that treatment, the more effective that treatment will be, so these new trials will help to test that.”
Who is eligible?
“Anybody who is infected with COVID-19, and is within eight days of onset of symptoms, is eligible — along with anyone who has had very close household contact with someone diagnosed with COVID-19 within 96 hours of exposure,” Heath said. To learn more about this and other clinical trials at UAB, call 205-996-4099 to determine if you are eligible to enroll.
What is involved?
The trials will consist of two clinic visits — an initial screening visit and a plasma treatment. After that, “they will be monitored closely at home and given a pulse oximeter” to measure blood oxygenation levels, Heath said. If the participant’s disease progresses to the point where they need hospitalization or other care, they will receive the same treatments as any other patient.
The treatment takes about 90 minutes, Heath said.
If you have already been diagnosed with COVID-19 and recovered, can you contribute to this research?
“Yes, absolutely,” Heath said. “We are very thankful to all the people who have donated plasma already. Given the recent increases in COVID-19 diagnoses and hospitalizations in Jefferson County, our need for convalescent plasma continues. Anyone recently infected who has recovered could potentially be a donor and help someone else.”
Donors neededConvalescent plasma donors must have a confirmed diagnosis of COVID-19 and have been symptom-free for 28 days. “Many people who are interested in donating plasma will be eligible,” Heath said. To volunteer as a convalescent plasma donor, call 205-996-4099. |
A single plasma donation can be used to treat two other patients with convalescent plasma therapy.
“We had a case at Children’s Hospital where we gave plasma to a young baby and several members of that family donated plasma afterward. They paid it forward.”
Anyone who has been hospitalized with a COVID-19 diagnosis or had moderate to severe illness may have developed antibodies, Heath said. “We would be interested in having them reach out to us directly at 205-996-4099 to find out if they are eligible to donate.”