 Written by Matt Windsor
Written by Matt Windsor
Media Contact: Bob Shepard, shep@uab.edu
When you hear the phrase “good genes,” you probably picture a supermodel like Kate Upton, or a sports superstar like Washington Nationals slugger Bryce Harper. People, in other words, who may have worked hard for their success, but were blessed with some helpful genes as well.
Superstar geneticist Haydeh Payami, Ph.D., has spent the past two decades searching for a different type of DNA. In a series of studies she calls “fishing expeditions,” Payami has been trawling the genome for bits of DNA that can help explain the mysterious patterns seen in Parkinson’s disease: Why do smokers have a much lower risk of getting the disease? What role does the immune system play in Parkinson’s? Why do some people get the disease in their teens, while it appears in others in their late 80s?
She has already had several intriguing catches. If you have the right combination of genetic variations, she explains, a few cups of coffee per day could reduce your Parkinson’s risk by an astounding 87 percent. With other sets of genes, a nicotine patch — or some daily ibuprofen, or probiotic pills — could do the trick. “If you could identify people who are genetically susceptible and tell them what to do, or what to avoid, maybe you can prevent the disease from happening,” Payami said.
It’s a perfect example of the potential of personalized medicine. But there’s more. Payami is now expanding her work to Alzheimer’s disease, and the same techniques could revolutionize the study and treatment of many other conditions.
The attractions of Alabama
Payami has a track record of success that includes several groundbreaking discoveries in Parkinson’s research. In 1994, “when the entire scientific and medical community thought Parkinson’s disease was purely environmental,” she said, her lab was the first to find a genetic component to the disease. “We are now at gene number 28 and counting.” Payami is the founder and leader of the NeuroGenetics Research Consortium, which has amassed 4,000 genetic samples and the most unique Parkinson’s data set of its kind on the planet. But to translate her findings from the lab to everyday life, she needs more samples, faster gene sequencing tools, more powerful computers and more scientific collaborators. And that’s why she left an enviably stable research position in New York, and a killer condo overlooking the Hudson River, for a new life in Alabama.
When David Standaert, M.D., chair of UAB’s Department of Neurology, brought Payami to Birmingham for a recruitment visit, he asked her repeatedly what it would take to get her to stay. “Finally, I said, everyone makes fun of me because in my CV I have written, ‘My 30-year goal is to have a prevention for Parkinson’s disease,’” Payami said. “I told him, ‘If you make that go to 15 years, I’ll come.’ And I think he did.”
With a new joint appointment at UAB and Huntsville’s HudsonAlpha Institute for Biotechnology — she is a professor of neurology at UAB and a faculty investigator at HudsonAlpha — Payami is doubling her DNA collection, and tapping into the latest genetic sequencing and analysis systems. She will add 2,000 patients and 2,000 healthy controls from UAB’s renowned Parkinson’s clinics, and have access to the world-class machines and analysts in HudsonAlpha’s Genomic Services Laboratory. “That will give us the power to do what we need to do,” Payami said. (Learn more about the Genomic Services Lab in this related story.)
Payami and Parkinson’s, by the numbers
Six things to know about Haydeh Payami: She left her native Iran for the United States at age 19, a few years before the 1979 revolution, with one suitcase and not a single friend or relative in America. She has a great deal of energy. She carries her life’s work with her in two large freezers and a Tupperware container. She does not lack confidence. She has a master plan (several, actually) to stop Parkinson’s. She loves her daughter.
Six things to know about Parkinson’s: It is caused by a progressive loss of dopamine-producing brain cells, which affects movement and other bodily functions, although the exact causes of this cell death are still unexplained. It affects around 1 million Americans, and 60,000 more are diagnosed each year. The main treatment, l-dopa, was developed 50 years ago; even though it can ease the symptoms of Parkinson’s, it does nothing to stop the progression of the disease. In the past decade, a series of “neuroprotective” drugs has generated excitement; but all have failed in clinical trials.
The good news is that these drugs aren’t necessarily failures, Payami says. She has a plan to resurrect at least some of them (more on that later). But there are three cheap, widely available drugs that seem to be able to prevent and even treat Parkinson’s: caffeine, nicotine and over-the-counter anti-inflammatory drugs.
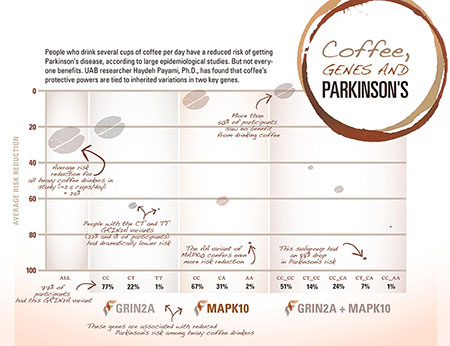 Click to enlargeThe protective power of a cup of coffee
Click to enlargeThe protective power of a cup of coffee
Payami makes her point with a jaw-dropping chart. “If you just ask people how much caffeine they drink and map that, you see that the risk for Parkinson’s drops by about 30 percent in heavy caffeine drinkers,” she said. It’s a pattern that epidemiologists have found in several studies, she points out. But Payami’s lab has been able to explore this relationship in an entirely new way. Her team scanned 7 million DNA variants from the genomes of 1,458 patients with Parkinson’s and 931 healthy matched controls, looking for genetic variations that set apart the heavy coffee drinkers who were protected against Parkinson’s from everyone else. (“Heavy” consumption, in this case, was around 2.5 cups per day and higher for 25 years.)
Her first hit was a gene called GRIN2A, out on chromosome 16. The most common form of the gene — the one that most of us have — doesn’t have a protective effect. But somewhere between a fifth and a quarter of patients in her sample have a mutated form of GRIN2A that dramatically alters their chances of getting Parkinson’s. “Their risk drops by 60 percent,” Payami said. Not only is this a fascinating finding for Parkinson’s researchers, but it has much larger implications, Payami points out. “This is the first demonstrated gene-environment interaction for any disease to come out of the modern whole-genome analysis.”
How it might work
What could be behind that protective effect? GRIN2A produces a receptor for glutamate, an important signaling chemical in the brain. “Balancing glutamate on neurons is critical for neuronal toxicity,” Payami said. “And glutamate toxicity is one of the suspected mechanisms in the cell death we see in Parkinson’s. So you can imagine that if you have a problem with the receptor, your risk will be higher.”
After GRIN2A, Payami’s team found another link in the coffee connection: a gene called MAPK10. They discovered two protective variants in this case. One MAPK10 mutation, found in 31 percent of her study samples, confers a 60 percent drop in Parkinson’s risk; the other, found in only 2 percent of samples, brings a 75 percent risk reduction.
MAPK10 is an apoptosis gene — it helps regulate programmed cell death, the beneficial process that clears out damaged or unhealthy cells from the body. But apoptosis can go awry in several diseases, including Parkinson’s. “In animal studies, mutating MAPK10 completely protects against Parkinson’s,” Payami said.
Who might benefit?
When Payami combined the data, she found that people who had the most common variants of both GRIN2A and MAPK10 — which is about 50 percent of those studied so far — received no Parkinson’s protection from coffee drinking. Another 40 percent of the people she studied had mutations that cut their risk in half. “And then there’s this lucky 7 percent, who have about an 87 percent risk reduction” when they consumed heavy quantities of caffeine, Payami said.
These are the people who should be drinking lots of coffee — or consuming a prescribed, regular dose of caffeine in pill form, perhaps. “Many people will see no benefit; but for some people, it will drastically reduce their risk of getting Parkinson’s,” Payami said. “We have to figure out the right dose, because too much caffeine can be harmful, even fatal.”
| “If you divide people between those who smoked regularly and those who never smoked, there’s clearly a 25 percent lower risk of Parkinson’s disease among the smokers.” |
Protection from nicotine, anti-inflammatory drugs
Payami’s team has uncovered the same pattern among smokers. “If you divide people between those who smoked regularly and those who never smoked, there’s clearly a 25 percent lower risk of Parkinson’s disease among the smokers,” Payami said. But just as with caffeine, her team found large differences based on variations in a specific gene: in this case, SV2C. They have also identified a long, non-coding RNA behind the protective effect seen among people who regularly take anti-inflammatory drugs.
Two major, phase 3 clinical trials in humans are underway to gather more data on the effects of caffeine and nicotine on Parkinson’s. “These trials had already started when we discovered the genes,” Payami said; but she is now part of both. In one case, she sent an email to the principal investigator to ask if she could join in; in the other, “the PI came up to me to suggest a collaboration after a talk that I gave,” she said. The investigators leading both trials are now “drawing blood on their patients, and we’re going to genotype them here.”
If Payami’s genetic findings are borne out in clinical trials, it will be relatively simple to implement them in everyday medical practice, she notes. “All you’re doing is taking a saliva sample,” Payami said. “It’s a test that, if you’re doing it one at a time costs 50 cents, and if you’re doing it massively it’s less than a penny per person.”
Here’s where you come in …
The current clinical trials are testing caffeine and nicotine as treatments for Parkinson’s symptoms, Payami notes; they’re not examining preventive effects.
 Payami has an ambitious plan to do that prevention trial, however. “We’d like to genotype tens of thousands of people who do not have the disease,” she said. “We want to follow them and see who is going to get the disease when, and who is not — and whether that is related to how much they smoked or did not smoke and how much caffeine they took or what kinds of diseases they had, whether they are using inflammatory drugs or not, and so on.”
Payami has an ambitious plan to do that prevention trial, however. “We’d like to genotype tens of thousands of people who do not have the disease,” she said. “We want to follow them and see who is going to get the disease when, and who is not — and whether that is related to how much they smoked or did not smoke and how much caffeine they took or what kinds of diseases they had, whether they are using inflammatory drugs or not, and so on.”
After she discovered the two caffeine-related genes, “I started to call this my Starbucks project,” after a perfect potential sponsor, she says with a laugh. Now, with the addition of nicotine patches and anti-inflammatory agents to the possible preventive agents, the pool is much larger. “If companies bought into this, we could advertise it nationally and recruit tens of thousands of people to join,” she said. “They would each send in a saliva sample, and we could sequence their whole genome from that. Then they could go online every six months and update us on how they’re doing — as well as lifestyle factors, what they’re taking as far as inflammatory drugs, how much caffeine they are drinking, whether they are smoking.”
With a large enough sample, “you could have all the answers in five years, plus another few years to do the math and develop the algorithm that will say who would most benefit from caffeine, nicotine and anti-inflammatory drugs,” Payami said.
Danger in the “second brain”
In another project, Payami’s lab is studying the link between Parkinson’s disease and the community of microbes in our intestines: the microbiome. The connection, she explains, is a protein called alpha-synuclein. “Deposits of alpha-synuclein in the brain are the hallmark of Parkinson’s disease,” Payami said. “But alpha-synuclein deposits first show up in the gut.” Because neurons reach all the way from the brain down to the gut, some researchers hypothesize that alpha-synuclein somehow becomes dysregulated in the intestines, then travels upward through the nervous system until it reaches the brain. “One clue is that constipation is one of the first signs of Parkinson’s disease,” Payami said.
She is now working with renowned microbiome expert Rob Knight, Ph.D., a biologist at the University of California-San Diego, and researchers at Emory University on a pilot study of 200 Parkinson’s patients and 150 controls. “It’s nearly complete,” she said. “We’re looking for a Parkinson’s signature in the microbiome of the gut. Then we’ll do a full-blown study with the 2,000 patients and 2,000 controls from UAB.”
Immunity and Parkinson’s
One of the Payami lab’s most significant discoveries so far revealed the role of the immune system in Parkinson’s. “Since the 1980s, people have been saying that the immune system is involved in Parkinson’s disease” in a causative role, Payami explained. “But others have said, ‘No, the immune system activity we are seeing is a consequence of the Parkinson’s, not the cause.’”
| One of the Payami lab’s most significant discoveries so far revealed the role of the immune system in Parkinson’s. “Since the 1980s, people have been saying that the immune system is involved in Parkinson’s disease” in a causative role, Payami explained. “But others have said, ‘No, the immune system activity we are seeing is a consequence of the Parkinson’s, not the cause.’” |
In one of her fishing expeditions, Payami’s team “pulled out HLA” — human leukocyte antigen, a group of genes that make the proteins that signal the immune system’s foot soldiers to destroy foreign cells. The discovery, published in Nature Genetics in 2010, “kind of nails the involvement of the immune system,” said Payami. She is now joining a UAB study led by neurology chair David Standaert on innate and adaptive immunity in Parkinson’s, and she plans to collaborate with HudsonAlpha faculty investigator Jian Han, Ph.D., a world-renowned expert in the immune repertoire, to search for other immune players in Parkinson’s.
Removing the trial and error of treatment
Genetic insights will also change the way that physicians treat Parkinson’s disease, Payami predicts. “Right now a patient comes in and it’s trial and error,” she said: “Will this drug work? At what dose? What kind of a drug cocktail do we make for them?”
Payami plans to join a study, led by Standaert, that is correlating patients’ genetic signatures with their response to l-dopa. Although this drug is still a cornerstone of Parkinson’s treatment, many patients develop an uncontrolled shaking, known as dyskinesia, after using l-dopa for anywhere from a few months to several years. Standaert “is trying to figure out if he can find a gene that makes some people develop dyskinesia where others don’t,” Payami said.
Ultimately, Payami wants to catalogue these gene-drug interactions for a wide range of Parkinson’s symptoms. “What I’m going for is you go in, get a finger prick, they put it on a slide and then slip it into a machine on the doctor’s desktop, and she can tell you, ‘Yes, you have Parkinson’s disease, and if we put you on l-dopa you’re going to develop hallucinations. So let’s put you on this other drug.’
“Or,” she continues, “my daughter can get tested and figure out if she’s at risk, and if she is, what can we do about it?”
Payami has several more active research areas, including this perplexing question: Why do some people develop Parkinson’s in their 20s, while others aren’t afflicted until their 80s? “What sets those people apart?” Payami wondered.
“If you could delay the age of onset by a few years, you could make a big difference,” she pointed out. Her lab has identified a gene variant that affects age of onset by eight years, but much more data is needed. With the additional patient samples from UAB and HudsonAlpha’s sequencing capabilities, “I want to do whole-genome sequencing of uncommon and rare variants affecting age of onset,” she said.
Delivering on the promise
Even as she forges ahead with Parkinson’s studies, Payami is also planning to reopen her investigations of the genetics of Alzheimer’s disease. “I started out in Alzheimer’s; but after the four major genes for Alzheimer’s were discovered, the field was at a standstill,” she said. “That’s why I moved to Parkinson’s.”
But now, with the advent of genome wide association studies, and the analytic tools necessary to decipher them, “I can go back,” she said. “I would like to take pharmacogenomics there, to look at genetic factors related to drug response in Alzheimer’s.”
The combination of collaborators, technology and expertise available at UAB and HudsonAlpha is a “dream come true” with potential applications to a host of diseases, Payami said. “This isn’t something you could accomplish at one institution; it’s going to take everyone working together to get a project like this done.”
The effort required is significant, but successful outcomes will be felt worldwide, she adds — and at home. “If we can have a prevention for Parkinson’s in 15 years, my daughter will be 39 and it will be perfect timing,” Payami said. “And I will be 74 and need better treatments. To me, this is a marriage of two fabulous opportunities. Now I have to deliver.”