 Victor ThannickalA new study from the University of Alabama at Birmingham highlights why fibrotic diseases — diseases that feature fibrosis or scarring of organ tissue — are typically associated with aging.
Victor ThannickalA new study from the University of Alabama at Birmingham highlights why fibrotic diseases — diseases that feature fibrosis or scarring of organ tissue — are typically associated with aging.
Fibrosis, the formation of fibrous scar tissue in response to injury, is part of the normal healing process. In young animals or people, scars resolve or fade away over time and are replaced by newly grown healthy tissue. In older subjects, the scars do not resolve or fade, and scar tissue can build up. In organs such as the heart, lungs, kidneys or liver, the buildup of scar tissue can interfere with normal function, with potentially devastating results.
In findings published April 9 in Science Translational Medicine, the UAB team describes the mechanism that contributes to persistent fibrosis in the aged, and suggests targets that may help reverse the buildup of scar tissue.
“The findings presented here represent the first understanding we have of why fibrosis is more prevalent in older individuals,” said Victor J. Thannickal, M.D., professor and director of the Division of Pulmonary, Allergy and Critical Care Medicine at UAB and the study’s senior author. “With a better understanding of the pathway that prevents the normal resolution of scar tissue in the elderly, we can look for new therapies that may help prevent or slow the progression of fibrotic diseases.”
One fibrotic disease in particular is the focus of Thannickal’s laboratory. Idiopathic pulmonary fibrosis is a progressive and fatal lung disease with no effective treatment or cure. Often brought on by environmental triggers such as smoking, IPF usually worsens with age. Progressive scarring within the air sacs of the lungs blocks them from performing their function of exchanging oxygen for carbon dioxide. Patients in effect suffocate from within.
“In response to lung injury, cells called myofibroblasts are recruited to facilitate healing. An oxidant-generating enzyme, NADPH oxidase-4 (Nox4), promotes the formation of myofibroblasts. As normal healing proceeds and the myofibroblasts’ job is completed, they undergo apoptosis, or programmed cell death, which allows the scar tissue to resolve and normal healing to occur,” said Louise Hecker, Ph.D., assistant professor and first author of the study.
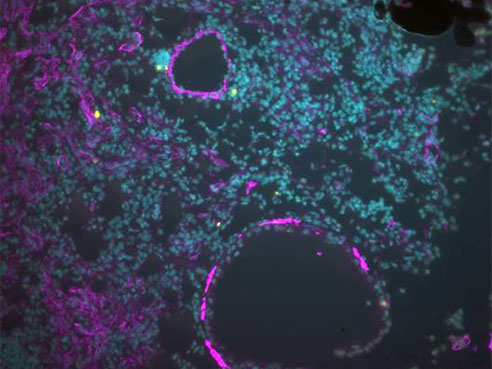 Image of injured lungs from young mice with resolving fibrosis showing dying myofibroblasts (in purple; with yellow denoting apoptotic cells). In old animals, this essential process of apoptosis does not occur, resulting in the accumulation of myofibroblasts and progressive fibrosis.
Image of injured lungs from young mice with resolving fibrosis showing dying myofibroblasts (in purple; with yellow denoting apoptotic cells). In old animals, this essential process of apoptosis does not occur, resulting in the accumulation of myofibroblasts and progressive fibrosis.
Hecker says the study shows that the ability to resolve scar tissue was markedly diminished in older mice. This inability to resolve fibrosis was attributed to myofibroblasts’ becoming resistant to apoptosis and persisting within injured tissue. In older mice, this resistance to apoptosis is the result of a deficiency in activating the antioxidant response transcription factor, nuclear factor-like 2, or Nrf2. This imbalance between Nox4 and Nrf2 was observed in the animal model, as well as in human IPF fibroblasts and lung tissue.
“Fibrosis affects organ systems other than the lungs, and aging is often a risk factor for the disease and its progression,” said Thannickal. “This research is exciting in that we now understand how age affects fibrosis, and we now have two targets to reduce or control fibrosis. We can look for ways to better activate or to boost Nrf2, or we can look for ways to inhibit Nox4 directly.”
Hecker and Thannickal’s team is developing novel drug candidates to target Nox4.
Human fibrotic disorders are estimated to contribute to 45 percent of deaths in the United States. IPF affects 200,000 people in the United States and 5 million worldwide.
Funding for this study was provided by the National Institutes of Health and the Department of Veterans Affairs.