 From left: Salpy Pamboukian, George "Mac" McAllister, Pat McAllister and James Kirklin.George “Mac” McAllister had already endured two heart attacks, quadruple-bypass surgery, myopathy, ischemia of the heart and a congestive heart failure diagnosis by the time he arrived at the University of Alabama at Birmingham Heart & Vascular clinic in October 2014. Actually, McAllister, a Hayden, Alabama, native, had experienced all of those things, including the congestive heart failure diagnosis, some five and a half years earlier.
From left: Salpy Pamboukian, George "Mac" McAllister, Pat McAllister and James Kirklin.George “Mac” McAllister had already endured two heart attacks, quadruple-bypass surgery, myopathy, ischemia of the heart and a congestive heart failure diagnosis by the time he arrived at the University of Alabama at Birmingham Heart & Vascular clinic in October 2014. Actually, McAllister, a Hayden, Alabama, native, had experienced all of those things, including the congestive heart failure diagnosis, some five and a half years earlier.
So it wasn’t a surprise when UAB physicians told McAllister he had finally reached end-stage congestive heart failure and would need a transplant. McAllister’s health had declined so much in the months leading up to his October visit, however, that he wasn’t a candidate for transplant and would have a difficult time becoming a candidate without marked improvement.
“In short,” McAllister said, “my heart would not have held out long enough for a transplant without some kind of assistance.”
McAllister was admitted to UAB Hospital on Dec. 1 after a two-week outpatient evaluation for a heart transplant, and on Dec. 17, he became the first person outside Japan to receive the Evaheart Left Ventricular Assist Device, a potentially more physiologic L-VAD device than others currently available to help bridge patients to transplant.
McAllister’s milestone surgery began the US Pivotal Trial, which will include up to six VAD clinical sites along with UAB and enroll up to 20 patients. UAB was chosen to participate in the Evaheart trial in part because of its international prominence in the field. Salpy Pamboukian, M.D., associate professor of medicine and section chief of Advanced Heart Failure, Transplantation and Pulmonary Vascular Disease in the School of Medicine, is the principal investigator for the UAB site. The current phase of the study is getting patients successfully to transplant, and Pamboukian says McAllister is on his way.
“Mr. McAllister is a pioneer,” Pamboukian said. “It takes a lot of courage to be the first person to go through this kind of experience. He was unwavering, and the best news is that he has done so well since the implant that he is now a candidate for a heart transplant.”
Heart-assist devices, or VADs, are implantable mechanical pumps that support blood flow in patients with severe heart failure. Implantable VADs can sustain the heart function of a patient for years and essentially serve either as a bridge-to-cardiac transplantation or for permanent use as a destination therapy.
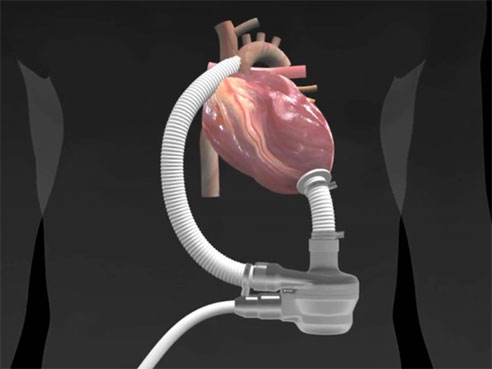 Click to watch the video.The Evaheart continuous-flow mechanical support device is similar to two others available in the United States — the HVAD and the HeartMate II. UAB surgeons implant approximately 40 of these devices per year and have implanted more than 430 total VAD pumps since 1989. UAB ranks among the leading hospitals in the United States for these therapies.
Click to watch the video.The Evaheart continuous-flow mechanical support device is similar to two others available in the United States — the HVAD and the HeartMate II. UAB surgeons implant approximately 40 of these devices per year and have implanted more than 430 total VAD pumps since 1989. UAB ranks among the leading hospitals in the United States for these therapies.
The Evaheart presents different challenges for surgeons than the other L-VAD devices, however. One of those is that the Evaheart is larger, says James Kirklin, M.D., professor and director of the Division of Cardiothoracic Surgery, who, assisted by William Holman, M.D., professor of surgery, implanted the Evaheart into McAllister.
Another challenge is that the surgery is more complex and longer, in large part because the device requires surgeons to develop a pocket in the muscles of the abdominal wall much larger than that required for other current pumps.
“The downside of the device is that everything is a bit more cumbersome,” Kirklin said. “The driveline is bigger. The VAD is bigger. However, the larger size accommodates a water-cooling system, which is unique to the Evaheart pump. Part of this experiment with the device is to see if having everything a bit larger and bulkier translates into fewer adverse events.”
That has been the case in Japan, where the system is manufactured and where it has been commercially available since 2010. The Evaheart has shown to have the advantages of a higher pump-flow capacity, augmentation of the native pulsatility of the heart and a lower risk of gastrointestinal bleeding in the Japanese clinical experience.
“The output from this pump is one of the highest we have seen,” Kirklin said. “Mr. McAllister had a tremendous cardiac output after surgery. And there is some evidence that, because of the increased pulsatility, the fragility of small vessels in the brain and the GI tract may be more favorably affected. That’s what the Food and Drug Administration is trying to determine with this trial.”
| Under the U.S. Investigational Device Exemption Clinical Trial, Evaheart is appropriate for bridge-to-transplant patients. Until clinical trial data becomes available, it is considered too early to discern additional characteristics of the “ideal” Evaheart patient. Evaheart, Inc., believes the study will demonstrate that the pump is ideal for candidates who have a larger body size, prior history of GI bleeding or mild end-organ dysfunction. |
“One of the things that we worry about with L-VADs is blood clots’ forming within the pump, something that is a known complication with other pumps,” Pamboukian added. “If we can minimize this type of long-term complication, it could be viewed as a destination therapy for those who for whatever reason could not receive a transplant.”
Although McAllister is the first person outside Japan to receive the Evaheart, more than 130 Japanese patients have been implanted with the device. Sixty-seven of those patients are still on the device, and 19 patients have been transplanted. The total accumulated support time for these patients thus far is 203 years, with an average support time of 693 days, or 1.89 years, according to Evaheart, Inc., the company who will distribute the device upon market clearance.
Under the U.S. Investigational Device Exemption Clinical Trial, Evaheart is appropriate for bridge-to-transplant patients. Until clinical trial data becomes available, it is considered too early to discern additional characteristics of the “ideal” Evaheart patient. Evaheart, Inc., believes the study will demonstrate that the pump is ideal for candidates who have a larger body size, prior history of GI bleeding or mild end-organ dysfunction.
Both Pamboukian and Kirklin were impressed that McAllister researched the Evaheart and asked to be part of the trial.
“My wife, Pat, and I prayed about it, and we decided we wanted to do this,” McAllister said. “For our own purposes we wanted to, perhaps, have a better unit installed — and the Japanese trial turned out really well, which gave us hope this pump was better.
“And maybe the overriding factor with me and my final decision was that, if this device can work and we can get it FDA-approved, then hopefully down the road many will benefit from it. I really wanted to be a part of making that happen. It’s not that I’m a brave person or that there is really anything special about me, but that really weighed heavily in my decision. I just thought if we could get the ball rolling here in the United States, maybe we could get on down the road toward getting this approved and hopefully moving into a brave new future with the L-VAD and folks benefiting from the devices even more than they do now.”
Enrollment in the trial will continue through 2015, as will ongoing consultation with the FDA. Evaheart, Inc., plans to expand the trial to 40 sites and 140 patients upon completion of the pivotal safety group.