Study Design
We conducted a multi-center, investigator-initiated study to determine i) the uptake of HPV vaccine and the determinants of non-initiation via survey, with comparison to nationally representative population data; and ii) a phase II single arm clinical trial to evaluate the safety and immunogenicity of the HPV vaccine in young cancer survivors (NCT01492582). Study participants included cancer survivors 9-26 years of age and 1-5 years post-cancer therapy; those without prior history of HPV vaccine initiation were invited to participate in the clinical trial. We administered the 3-dose HPV vaccine series over 6 months, collected serum for antibody titers at baseline and Month 7, and collected safety data.
Results
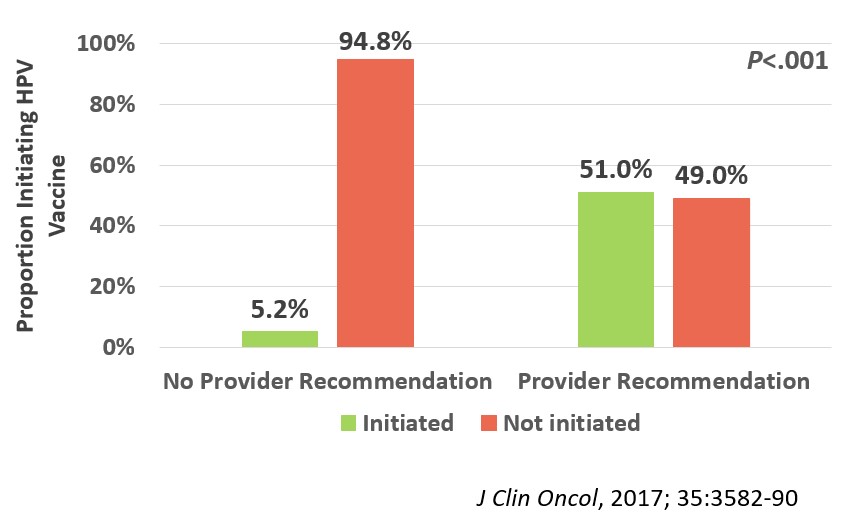
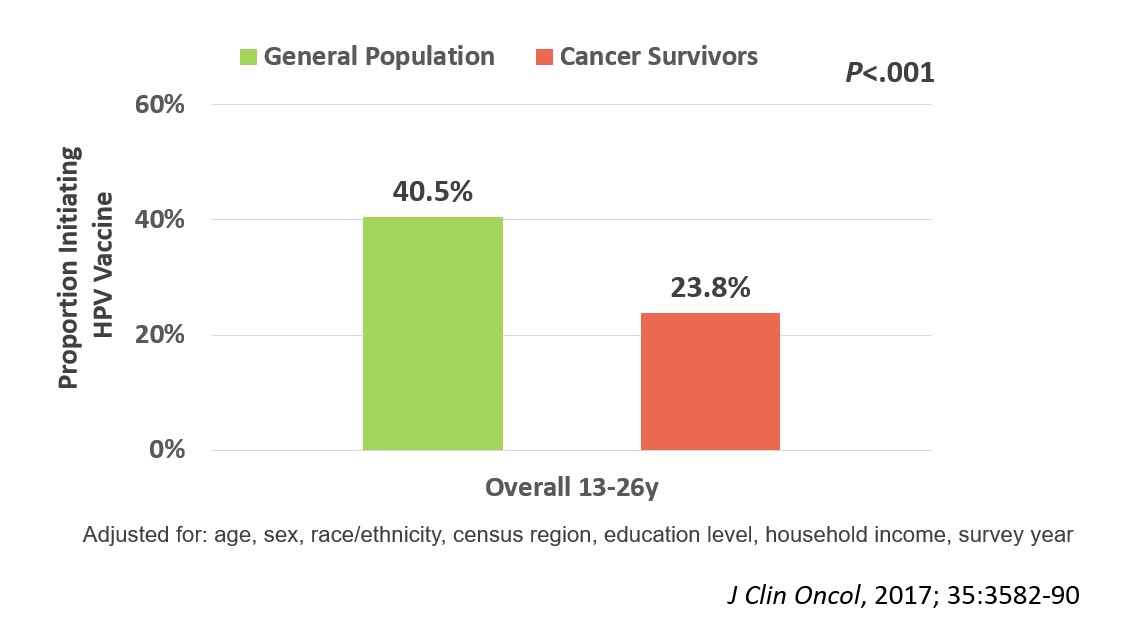 We enrolled 962 evaluable participants in the survey portion of the trial (median age 16.3 [range, 9-26]y; median time off therapy 2.8 [range, 1-5]y; 66% non-Hispanic white; 36% leukemia; 19% with history of blood or marrow transplant). After adjusting for age, sex, race/ethnicity, census region, education level, household income, and survey year, we found that HPV vaccine initiation rates were significantly lower (23.8%) among 13-26-year-old cancer survivors than their general population comparison group (40.5%; p<.001; Figure 1). The strongest predictor of HPV vaccine non-initiation among the survivors was lack of healthcare provider recommendation for the vaccine (Odds Ratio, 10.8; 95% confidence interval, 6.5-18.0, p<.001; Figure 2).
We enrolled 962 evaluable participants in the survey portion of the trial (median age 16.3 [range, 9-26]y; median time off therapy 2.8 [range, 1-5]y; 66% non-Hispanic white; 36% leukemia; 19% with history of blood or marrow transplant). After adjusting for age, sex, race/ethnicity, census region, education level, household income, and survey year, we found that HPV vaccine initiation rates were significantly lower (23.8%) among 13-26-year-old cancer survivors than their general population comparison group (40.5%; p<.001; Figure 1). The strongest predictor of HPV vaccine non-initiation among the survivors was lack of healthcare provider recommendation for the vaccine (Odds Ratio, 10.8; 95% confidence interval, 6.5-18.0, p<.001; Figure 2).
 Four hundred thirty six survivors enrolled on the vaccine clinical trial and received at least one dose; 378 (87%) of these survivors completed the 3-dose series per protocol and had evaluable immunogenicity data. We found that vaccine immunogenicity was similar in cancer survivors and the general population. Cancer survivors reported significantly fewer injection site-related adverse events, similar rates of fever, and significantly more nausea and fatigue than population comparisons. When we evaluated factors associated with lower immunogenicity, 15% of the cohort met the pre-defined criteria (Month 7 geometric mean titers for HPV type 16 and/or 18 less than half the mean of the corresponding general population comparison group). The only factor significantly
Four hundred thirty six survivors enrolled on the vaccine clinical trial and received at least one dose; 378 (87%) of these survivors completed the 3-dose series per protocol and had evaluable immunogenicity data. We found that vaccine immunogenicity was similar in cancer survivors and the general population. Cancer survivors reported significantly fewer injection site-related adverse events, similar rates of fever, and significantly more nausea and fatigue than population comparisons. When we evaluated factors associated with lower immunogenicity, 15% of the cohort met the pre-defined criteria (Month 7 geometric mean titers for HPV type 16 and/or 18 less than half the mean of the corresponding general population comparison group). The only factor significantly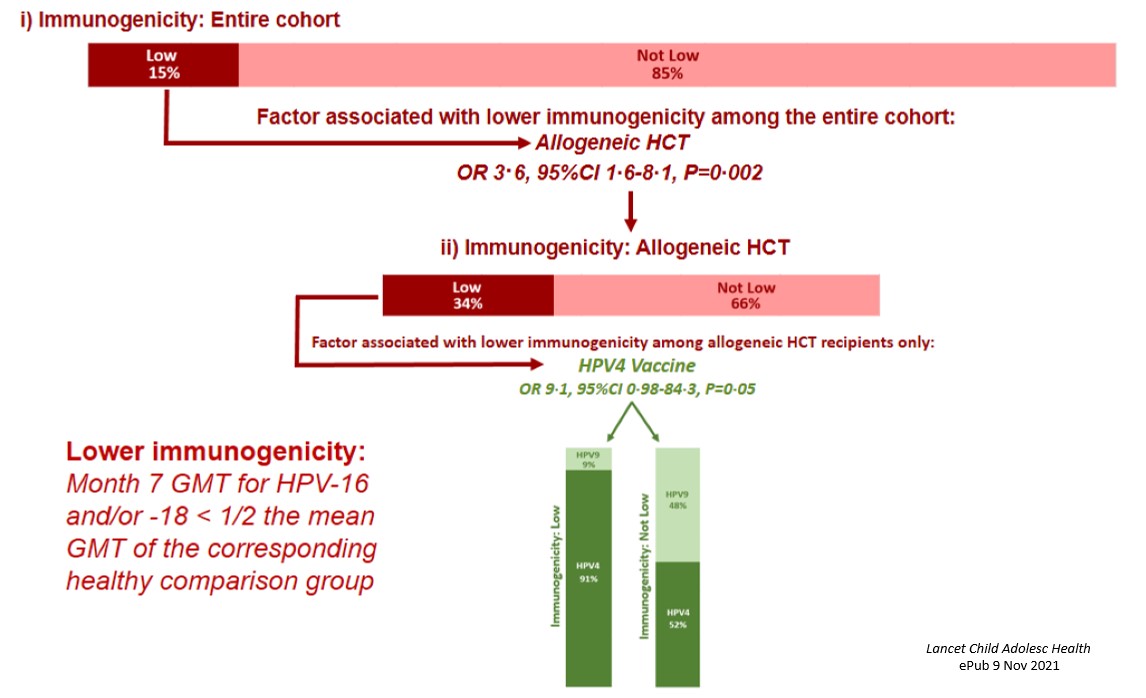 associated with lower immunogenicity was allogeneic transplant (34% of survivors of allogeneic transplant met the pre-defined criteria); among these, the only factor associated with low immunogenicity was receipt of the quadrivalent (compared with the nonavalent) HPV vaccine (Figure 3).
associated with lower immunogenicity was allogeneic transplant (34% of survivors of allogeneic transplant met the pre-defined criteria); among these, the only factor associated with low immunogenicity was receipt of the quadrivalent (compared with the nonavalent) HPV vaccine (Figure 3).
Conclusions and Future Directions
We found that uptake of the HPV vaccine is significantly lower in cancer survivors compared to the general population, and that lack of healthcare provider recommendation is significantly associated with vaccine non-initiation in cancer survivors. We also found that the HPV vaccine is safe and well-tolerated in cancer survivors, with an immunogenicity profile similar to the general population, and that lower immunogenicity was associated with receipt of the quadrivalent HPV vaccine (no longer in use in most countries) after allogeneic transplant. Findings from this study highlight the importance of healthcare recommendation for the HPV vaccine in cancer survivors, and for incorporating HPV vaccination into routine oncologic follow-up care, with the ultimate goal of preventing HPV-related subsequent neoplasms in cancer survivors.