 i2b2 is a self-service tool that enables researchers to access EHR data in the Cerner system. Dr. James J. Cimino, CCTS CoDirector and Director of the UAB Informatics Institute, is so sure researchers will benefit from learning how to use i2b2, he is running an i2b2 abstract contest. The grand prize for the best abstract is $1000, with two-second place prizes of $500. “But the real prize,” he said “is learning to access clinical data on your own.”
i2b2 is a self-service tool that enables researchers to access EHR data in the Cerner system. Dr. James J. Cimino, CCTS CoDirector and Director of the UAB Informatics Institute, is so sure researchers will benefit from learning how to use i2b2, he is running an i2b2 abstract contest. The grand prize for the best abstract is $1000, with two-second place prizes of $500. “But the real prize,” he said “is learning to access clinical data on your own.”
Still need convincing? Take inspiration from these i2b2 factoids:
- i2b2 has data on more than 1 million patients!
- There are 500 million rows of data!
- There are 140 million lab results!
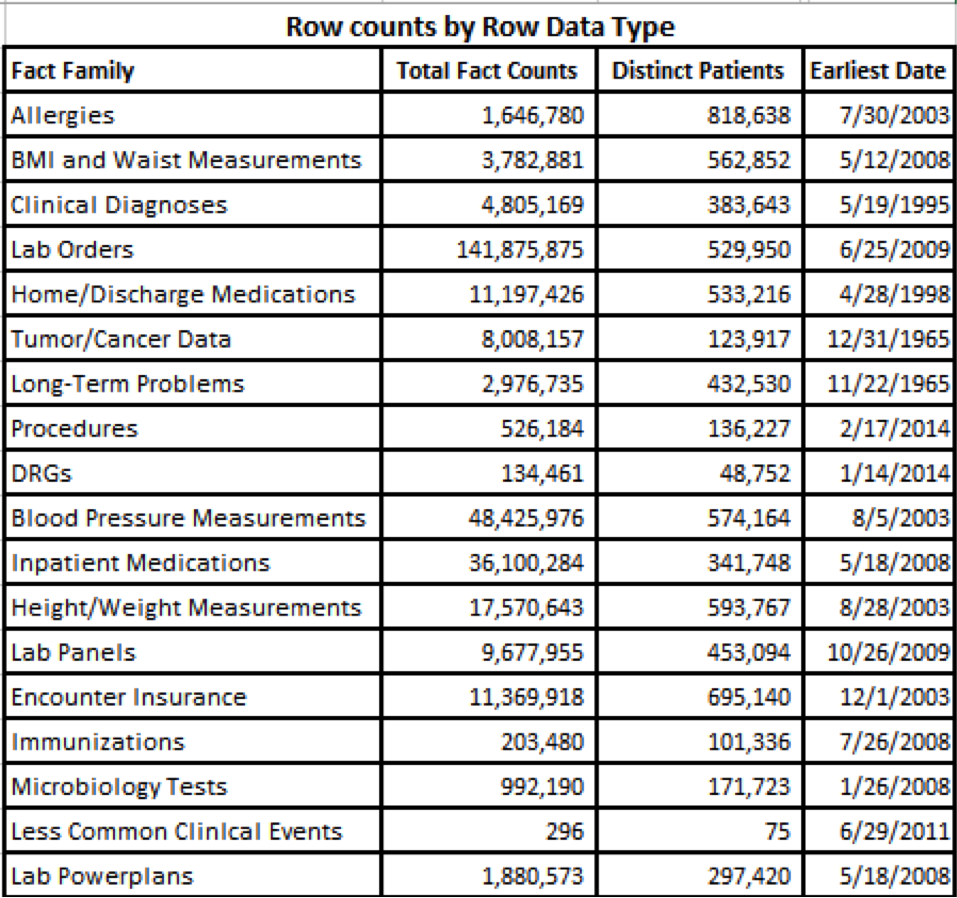
- From measurements to medications, covering diagnosis to discharge, researchers can query 18 i2b2 data types, with more on the way! (see Row Counts by Row Data Type)
- One does not need to be tech-savvy, the i2b2 interface is drag and drop.
- IRB exemption (for limited data set use) is automatically provided at the time of data download.
- Investigators can use i2b2 for generating hypotheses, estimating cohort size, exploring recruitment potential, and conducting simple data analyses suitable for correlational and retrospective studies.
- i2b2 access is available to all UAB investigators and is available to non-faculty with supervisor approval.
- i2b2 offers researchers a self-service solution to information needs that can be significantly faster than requesting data from Enterprise Data Warehouse analysts.
- CCTS and its partner the Informatics Institute offer several training options, including a hands-on class (see below for date/time/location and links to register), a video series, and a user manual.
- Requesting access is easy, here’s a link to the form:https://i2b2-uab.hs.uab.edu/webclient/uab_i2b2_Helper.php.
- The only prerequisites are: HIPPA and IRB Human Subject Protection training and acknowledgment of restrictions on the use of limited data sets (simple on-line acknowledgment of the data use agreement).
Register now for a December i2b2 training (registration is required):
When: 1:00-3:00 pm
Where: Cudworth Building, Room 305
Thursday, December 7 | Register
Tuesday, December 19 | Register
The i2b2 abstract contest offers a financial incentive for doing something that is already a win-win. Submit a one-page abstract with your findings (no p-value required) and you may win $500-1000—just in time for the holiday bills! Contest details are available online. Questions? Contact Informatics Institute Director Dr. James Cimino at
To learn more about the different types of data sets that CCTS Informatics enables via i2b2 or, for more complex queries, our Enterprise Data Warehouse analysts, see our Research Data Request page.
By Kate Matthews
More than 85 postdocs, trainees, grad students and faculty members attended the new Research Data Management Workshop, cohosted by CCTS, UAB Libraries, and the School of Health Professions. CCTS Co-Director of Biostatistics, Epidemiology, and Research Design (BERD) and Professor and Vice Chair of UAB Biostatistics David Redden, PhD, led the discussion on data management (DM) principles, including why it is important to consider the entire data lifecycle, and highlighted DM resources at UAB to help investigators manage their data.
“You wouldn’t build a house without laying a foundation first,” Redden began, “so one of the first things you should do before starting your research is to plan how you will manage the data lifecycle.” He defined data management as “a series of practices designed to maximize and protect the quality, utility, and completeness of an investigator’s research data.”
 The Data Lifecycle: The Good and The Bad
The Data Lifecycle: The Good and The Bad
Redden noted that ideally one considers every stage of the data lifecycle, which includes the following:
- Project planning
- Pro tip – Build a data dictionary that contains all your variables, how they were measured, how to record missing data, and acceptable values.
- Writing a data management plan (see key components below)
- Data acquisition and entry (i.e., naming conventions, software, and logic and range checks)
- Data quality control/data version control/metadata procedures
- Pro tip – Metadata is data about data. Develop a page or file that describes all of your research files, e.g., raw data, processed data, programs, images).
- Data analysis
- Pro tip – Use a syntax recorder for documentation, work with a methodologist who can program it.
- Publication and data sharing
- Pro tip – Building an archive increases reproducibility.
- Data preservation and archiving (i.e., both short- and long-term storage)
- Data reuse
A less comprehensive but more common approach—planning the project, acquiring and analyzing data, and publishing the results—can make it challenging to share or reuse data. Keeping all data in one office or repository with little documentation increases the chance that data will be lost. This is considered “the bad” approach to handling the data lifecycle.
Bad turns to ugly, Redden explained, when, in addition to the above, there is poor record management, a lack of a metadata file or data dictionary, no routine process for backing up, and not knowing who on the research team has the latest version of the data. This is where a well-thought-out management plan can save the day(ta).
Data Management Planning
The planning stage is key to data management, Redden said, mentioning several websites that provide good examples of such plans (see www.uab.edu/faculty/rdm for a UAB version or download a free data management plan template at DMPTool). Investigators should address the following in their plan:
- Data types
- Contextual details (Metadata)
- Storage, backup, and security
- Provisions for protection/privacy
- Policies for re-use, accessing, sharing, archiving, and preserving
Campus Resources
Redden ended with a quote from Benjamin Franklin, “An ounce of prevention is worth a pound of cure.” DM is labor intensive and requires constant attention, he said, but investing the time at the beginning stages of one’s research will pay dividends for years to come. He encouraged the audience to take advantage of research data management expertise at UAB, such as the free weekly BERD Drop-in Clinics offered by CCTS. Additional UAB DM resources are listed below.
- CCTS Research Commons (BERD) (
This email address is being protected from spambots. You need JavaScript enabled to view it. , 4-7442) - UAB Informatics Institute (
This email address is being protected from spambots. You need JavaScript enabled to view it. , 4-1958) - UAB Department of Medicine RedCap (
This email address is being protected from spambots. You need JavaScript enabled to view it. , 4-4357) - Lister Hill Library (
This email address is being protected from spambots. You need JavaScript enabled to view it. , 4-2231)
In case you missed the workshop, Dr. Redden’s slide deck and video recording are available here. Dr. Redden will reprise this topic at our next CCTS Forum on Wednesday, Dec. 6, where he and other experts will answer questions and highlight additional tools and techniques for research data management.
Want to hear more about a specific topic on research data management? Contact the CCTS at
 CCTS Director Dr. Robert Kimberly provided an “on ramp” to the CCTS at the Dept. of Obstetrics and Gynecology Grand Rounds.CCTS Director Dr. Robert Kimberly recently presented at the Dept. of Obstetrics & Gynecology Grand Rounds. After reviewing the CCTS mission and components, he touched on the myriad ways CCTS brings value to the clinical and translational research enterprise at UAB and beyond.
CCTS Director Dr. Robert Kimberly provided an “on ramp” to the CCTS at the Dept. of Obstetrics and Gynecology Grand Rounds.CCTS Director Dr. Robert Kimberly recently presented at the Dept. of Obstetrics & Gynecology Grand Rounds. After reviewing the CCTS mission and components, he touched on the myriad ways CCTS brings value to the clinical and translational research enterprise at UAB and beyond.
“Our job is to accelerate the flow of discovery into delivery of health care; train our workforce across all disciplines; engage with our communities; and improve the clinical and translational research process from discovery to delivery, keeping our focus on the ultimate goal of improving human health,” he said.
Below we spotlight some of our most impactful CCTS capacities, learning opportunities, and collaborative platforms. We encourage our readers to share this information with colleagues who might benefit from joining CCTS—hot links to these and other CCTS activities are also available in our Welcome letter.
Science Support
The CCTS works with investigators at any stage of a project, from nascent idea to publication of findings, to ensure the highest level of extramural success.
Project Panels
Our panel recipients enjoy a much higher chance of receiving a fundable grant score—our average is more than three times the NIH baseline funding rate.
Informatics Gateway
A special venue for project development with an emphasis on bio- and/or clinical informatics expertise in which investigators bring innovative ideas for discussion to explore opportunities and novel research directions that can be enabled through scholarly partnership and collaboration (in contrast to connecting investigators to service providers).
BERD Consults
Our biostatistics, epidemiology, and research design (BERD) experts work closely with any investigator in need of support, especially during the critical design and implementation stages of a research project. Drop by for one of our weekly clinics or request a one-on-one consult.
Grant Library
We work with investigators across our Partner Network to offer this compendium of award-winning grant writing samples. Our NIH samples showcase best practice at the meeting the latest federal rigor, reproducibility, and transparency requirements.
Funding
Through our annual Partner Network Multidisciplinary Pilot program, we seek to fund new interdisciplinary clinical and translational projects. Other avenues of support include our voucher program and unique mini-sabbatical opportunity.
Explore our Research Commons domain for more ways CCTS can help you sharpen your science.
Research Collaboration
The CCTS seeks to catalyze collaborative efforts in research, taking a team science approach whenever possible to synergize efforts and speed the pace of discovery and its translation into improved health care.
SHRINE Consortium
CCTS has joined with other CTSA Hubs to federate data from each institution, creating a region-wide resource that enables more powerful comparative effectiveness research, clinical trial feasibility analysis, and recruitment by researchers with i2b2 expertise.
Southeast Health Alliance for Research (SHARe)
This 12-member consortium builds on the strengths of our CCTS Partner Network to provide a transformed multisite trials platform featuring faster time-to-activation, better coordination, and a robust recruitment pool.
Explore our Partnerships domain to find other collaborative platforms available through CCTS.
Professional Development
The CCTS promotes the continuous development of a diverse, multidisciplinary research workforce. We offer trainings and educational programs suitable for wherever you are on the career arc, including learning activities tailored for K and T scholars.
Clinical and Translational Science Training Program (CTSTP)
Boost your career with this free six-month certificate program in the latest clinical and translational research competencies. Currently accepting applications--submit by 5pm on Wed. Nov. 22, 2017.
Case Studies in Mentoring
Whether you are a mentor or a mentee, this eight-week seminar series will help you more successfully navigate the challenges in professional relationships.
Accessing Clinical Data for Research with i2b2
Join our hands-on training with i2b2 to explore using UAB's electronic health record data for your research data needs. You can use i2b2 to determine study feasibility, explore hypotheses for clinical studies, identify potential participants and even win money! Check out the i2b2 contest.
Explore our Training Academy domain and Clinical Translational training page for more learning opportunities.
Clinical Research Support
The CCTS offers state-of-the-art settings for adult and pediatric research as well as a number of clinical research supports, including biospecimen handling and storage, clinically trained research staff, and bionutrition. We facilitate the research process by tailoring our supports to meet the individual needs of each investigator we assist.
Clinical Research Support Program (CRSP)
In addition to keeping clinical researchers and their teams current on the latest regulatory requirements, CRSP offers assistance with feasibility, recruitment, implementation, budget building, monitoring, and data management.
Explore our Clinical Translation domain to learn more.
Innovation
The CCTS supports innovation through many pathways, including the NSF-inspired I-Corps initiative, education through gamification, and the development of new drugs and devices.
Kaizen
Kaizen is a gamification platform the CCTS uses to develop trainings in core competencies. The most popular of these is our Rigor Reproducibility & Transparency (R2T) Kaizen game. Open to players across the CCTS Partner Network, R2T addresses the NIH requirement for all F, K, and T fellows to receive formal training in this area and can be used by institutional training programs (e.g., T32, K12) to fulfill this education expectation in a fun and innovative way.
Academic Drug Discovery and Device Development Program (AD4)
Through the AD4, CCTS offers investigators access to multidisciplinary project development teams that provide expertise tailored to a study's aims, including medicinal chemistry, high-throughput assay development, engineering, clinical application, and commercialization.
Explore our Special Modules domain to learn more about our latest innovative projects.
Community Engagement
Through its community engagement component, One Great Community, CCTS helps investigators leverage neighborhood and community relationships to build mutually respectful and beneficial programs underpinning service to our patients and populations.
Community Engagement Institute
The CEI is an annual learning and networking event that draws both researchers and community leaders to share best practices at the nexus of health, environmental renewal, and civil inclusion and empowerment. Our 4th annual event took place in October (for more, see “4th Annual CEI Advances Regional Health Equity Dialogue.”)
Regional Consortium
Drawing on the experience of the OGC, CCTS has organized a regional community engagement consortium, with active participation by several CCTS Partners. The overarching goal is to leverage institutional experiences to strengthen substantive community engagement across the Deep South, including developing and disseminating best practices in support of community engagement in different environments.
 On Wed. Nov. 1, more than 60 clinical investigators, research coordinators, and others interested in next-generation clinical trial research joined CCTS to toast the launch of OnCore, which will replace the current UAB clinical trials management system, SiteMinder. The implementation will take place in three waves, with trials in the Department of Medicine (DOM) the first to migrate into OnCore.
On Wed. Nov. 1, more than 60 clinical investigators, research coordinators, and others interested in next-generation clinical trial research joined CCTS to toast the launch of OnCore, which will replace the current UAB clinical trials management system, SiteMinder. The implementation will take place in three waves, with trials in the Department of Medicine (DOM) the first to migrate into OnCore.
In a brief presentation, CCTS Director Dr. Robert Kimberly highlighted the system’s many advantages, including centralized protocol tracking from startup to closeout, electronic ordering of clinical services, streamlined patient and billing processes, regulatory compliance support, and transmission of study and patient data to UAB’s electronic medical record. “OnCore will simplify our lives, making clinical trial management easier, faster, less prone to error. It will help us keep it all straight and manage trials in real time,” Kimberly said.
Kimberly also underscored the positive impact the system should have on recruitment, budgets, and patient safety. “Roughly half of all Phase III trials do not meet their recruitment goals—and we know that without enough recruits there isn’t sufficient statistical power to make reasonable conclusions from studies that are executed. OnCore will enable study teams to monitor and enhance recruitment with embedded mechanisms,” he noted. Support for more accurate capture and invoicing of billables could result in “nearly a doubling of the income from clinical trials.”
Patient safety will be improved as well—there is already a way to send a message from OnCore to UAB’s Cerner electronic health record (EHR). He encouraged emergency room clinicians to help scope what they need to see on the first page of a subject’s EHR to enable them to take clinical trial history into account, thus ensuring the “whole patient” is treated and clinical trial participants are recognized as such and receive appropriate care.
A panel of implementation experts and several super users answered attendees’ questions, which reflected concerns with the timing of the rollout, availability of OnCore training, accessing support via the HSIS help desk, the cost for using OnCore, and multisite study support. All attendees were provided with a handout of OnCore Frequently Asked Questions.
Dr. Kimberly reminded attendees that “implementation is a team sport—we need your help as OnCore is rolled out. Improvement is an iterative process, and we may hit a few speedbumps, but by working together we will get there.”
CCTS thanks its panel of OnCore experts: Dr. Cindy Joiner, Meredith Fitz-Gerald, Lisa Williams, Mark Marchant, John Sandefur, and Geoff Gordon.
In case you missed it, you can access the Nov. Forum video and slide deck from our CCTS Forum page. Mark your calendar for our Dec. Forum, which will take place on Wednesday, Dec. 6.
Congratulations to our new cohort of KL2 Scholars, whose appointments become official on Nov. 1, 2017.* They represent a variety of disciplines including neurology, nursing, orthodontics, and pulmonary critical care. Selected through a rigorous competitive process, KL2 scholars receive clinical and translational science development support for two years.
KL2s enroll in an educational program, usually the MSPH in Clinical and Translational Science, which imparts the CTSA core competencies as part of the curriculum. In parallel, they enter a research apprenticeship with a primary mentor who has an excellent training record and commits to extended close interaction with the scholar. The KL2 experience culminates in lead author manuscripts and an extramurally-funded research grant submission (e.g., R01).
Designed for junior faculty in a clinical or related discipline, the CCTS KL2 program aims to provide knowledge, experience, and perspective to candidates who show promise for becoming independent investigators. A key component of the program is protected time to allow both formal training and conducting hands-on research.
2017-18 KL2 Scholars
 Ejvis Lamani, DMD, PhD, assistant professor, UAB Dept. of Orthodontics
Ejvis Lamani, DMD, PhD, assistant professor, UAB Dept. of Orthodontics
Genetic Markers in Orthodontics
*Dr. Lamani’s appointment began June 15, 2017.
 Crystal Chapman Lambert, PhD, assistant professor, UAB School of Nursing
Crystal Chapman Lambert, PhD, assistant professor, UAB School of Nursing
Stress and Coping: Understanding Determinants of Adherence to HIV Care in African American Women Living with HIV
Mentors: Drs. Michael Mugavero, Janet Turan
 Jianmei Leavenworth, MD, PhD, assistant professor, UAB Dept. of Neurosurgery
Jianmei Leavenworth, MD, PhD, assistant professor, UAB Dept. of Neurosurgery
Identifying Immune Biomarkers for Malignant Gliomas to Oncolytic HSV Viroltherapy
Mentors: Dr. James Markert, Yancey Gillespie
 George Solomon, MD, UAB Division of Pulmonary, Allergy, and Critical Care Medicine
George Solomon, MD, UAB Division of Pulmonary, Allergy, and Critical Care Medicine
Nasal Cell Procurement in PCD Patients
Mentors: Drs. Steven Rowe, Stephanie Davis
 Chad Washington, MS, MPHS, MD, assistant professor, Neurosurgery, University of Mississippi Medical Center
Chad Washington, MS, MPHS, MD, assistant professor, Neurosurgery, University of Mississippi Medical Center
Sildenafil for the Treatment of Delayed Cerebral Ischemia following Subarachnoid Hemorrhage
Mentor: Dr. Alan Jones
*Dr. Washington’s appointment began April 1, 2017.
If you are interested in becoming a CCTS KL2 scholar, visit our Training Grant Programs web page.
