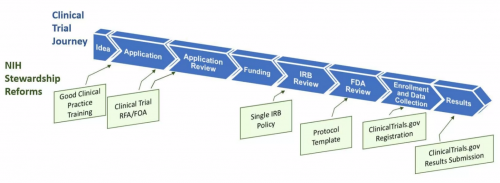
 Several federal agencies with oversight of clinical trials have announced changes that will impact nearly every stage of the clinical trial lifespan. The goal, as stated by the National Institutes of Health (NIH) Associate Director for Science Policy, Dr. Carrie Wolinetz, is “to enhance clinical trial stewardship.” This includes improving the quality and efficiency of clinical trials across their “lifespan.” There is a growing emphasis on the need to develop more innovative and robust clinical trial designs that will accelerate discoveries that advance human health.
Several federal agencies with oversight of clinical trials have announced changes that will impact nearly every stage of the clinical trial lifespan. The goal, as stated by the National Institutes of Health (NIH) Associate Director for Science Policy, Dr. Carrie Wolinetz, is “to enhance clinical trial stewardship.” This includes improving the quality and efficiency of clinical trials across their “lifespan.” There is a growing emphasis on the need to develop more innovative and robust clinical trial designs that will accelerate discoveries that advance human health.
CCTS will offer an in-depth analysis of federal reforms at its next Forum on Oct. 5th. A brief synopsis of changes per agency is provided below.
NIH
GCP training expectations for investigators and staff
As of January 1, 2017, NIH-funded investigators and staff who are involved in the conduct, oversight, or management of clinical trials must be trained in Good Clinical Practice (GCP). Documentation of initial and 3-year renewal of training will be required (NOT-OD-16-148).
Application process
NIH grant applicants requesting support for one or more clinical trials will need to respond to clinical trial-specific funding opportunity announcements (FOAs). NIH will no longer accept clinical trial applications submitted via “parent” FOAs (NOT-OD-16-147).
Scientific review
As of May 25, 2017, multi-site studies will be required to use a single Institutional Review Board (sIRB) of record for the ethical review of non-exempt human subjects research (NOT-OD-16-094).
Sharing research results with the public
To realize the benefits of a clinical trial, the findings must be available to the public as soon as possible after the trial has concluded. The new NIH policy sets the same ClinicalTrials.gov reporting expectations for all NIH-funded clinical trials whether or not they are subject to other complementary reporting rules, such as the new HHS Final Rule (see below) or the FDA reporting rule for phase 1 trials of drug and biological products and feasibility studies for devices (NOT-OD-16-149). Failure to comply can now result in suspended or restricted funding and potential civil monetary penalties.
NIH/Food and Drug Administration (FDA)
Clinical trial protocol template
To expedite both NIH and federal regulatory oversight processes, NIH and FDA are developing a clinical trial protocol template for phase 2 and 3 IND/IDE trials (Open Mike blog post).
Department of Health and Human Services (HHS)
Post-award management and oversight & sharing of trial data
HHS has promulgated a Final Rule requiring registering and submitting summary results information for certain clinical trials to ClinicalTrials.gov (42 FAR Part 11).
To learn more, including resources offered by CCTS that will help clinical trial PIs and their research teams meet these and other federal requirements, be sure to attend next week’s Forum and/or contact