 Rachel June Smith, Ph.D. (center) and members of the Neural Signal Processing and Modeling Lab team, including (left to right) Avinash Singh, Sahaj Patel, co-director Arie Nakhmani, Ph.D., Smith, Helen Brinyark and Noshin Tasnia.Photo by JENNIFER ALSABROOK-TURNER | UAB Marketing and CommunicationsSome 15 million people worldwide have seizures that cannot be controlled by medication. For many of those people, surgery holds the hope of a cure — if doctors can identify the tiny areas of their brains, unique to each patient, that are responsible for triggering the electrical storm of a seizure. But finding those areas, known as the “seizure onset zone,” is an expensive, time-consuming, frustrating process.
Rachel June Smith, Ph.D. (center) and members of the Neural Signal Processing and Modeling Lab team, including (left to right) Avinash Singh, Sahaj Patel, co-director Arie Nakhmani, Ph.D., Smith, Helen Brinyark and Noshin Tasnia.Photo by JENNIFER ALSABROOK-TURNER | UAB Marketing and CommunicationsSome 15 million people worldwide have seizures that cannot be controlled by medication. For many of those people, surgery holds the hope of a cure — if doctors can identify the tiny areas of their brains, unique to each patient, that are responsible for triggering the electrical storm of a seizure. But finding those areas, known as the “seizure onset zone,” is an expensive, time-consuming, frustrating process.
Patients can spend anywhere from several days to several weeks in an epilepsy monitoring unit with electrodes protruding from holes drilled in their skulls. This is intracranial EEG, and it is now often combined with electrical stimulation to map out the brain networks involved in a patient’s seizures. But even when doctors are convinced they have found the seizure onset zone, surgery is successful only about 50 percent of the time.
Computational neuroscientist Rachel June Smith, Ph.D., is exploring a new approach — inspired in part by a notorious episode of the Pokémon cartoon.
Smith, an assistant professor in the Department of Electrical and Computer Engineering in the UAB School of Engineering, joined the university in 2022 as one of the first major hires in UAB’s Neuroengineering and Brain-Computer Interface Initiative. Using linear algebra and engineering theory, paired with single-pulse electrical stimulation data, she is able to calculate the best way to push suspect brain tissue to reveal itself as an agent of chaos. She already has strong early patient data to demonstrate the method — and she might be able to do the same thing with a completely non-invasive test.
Story continues after infographic
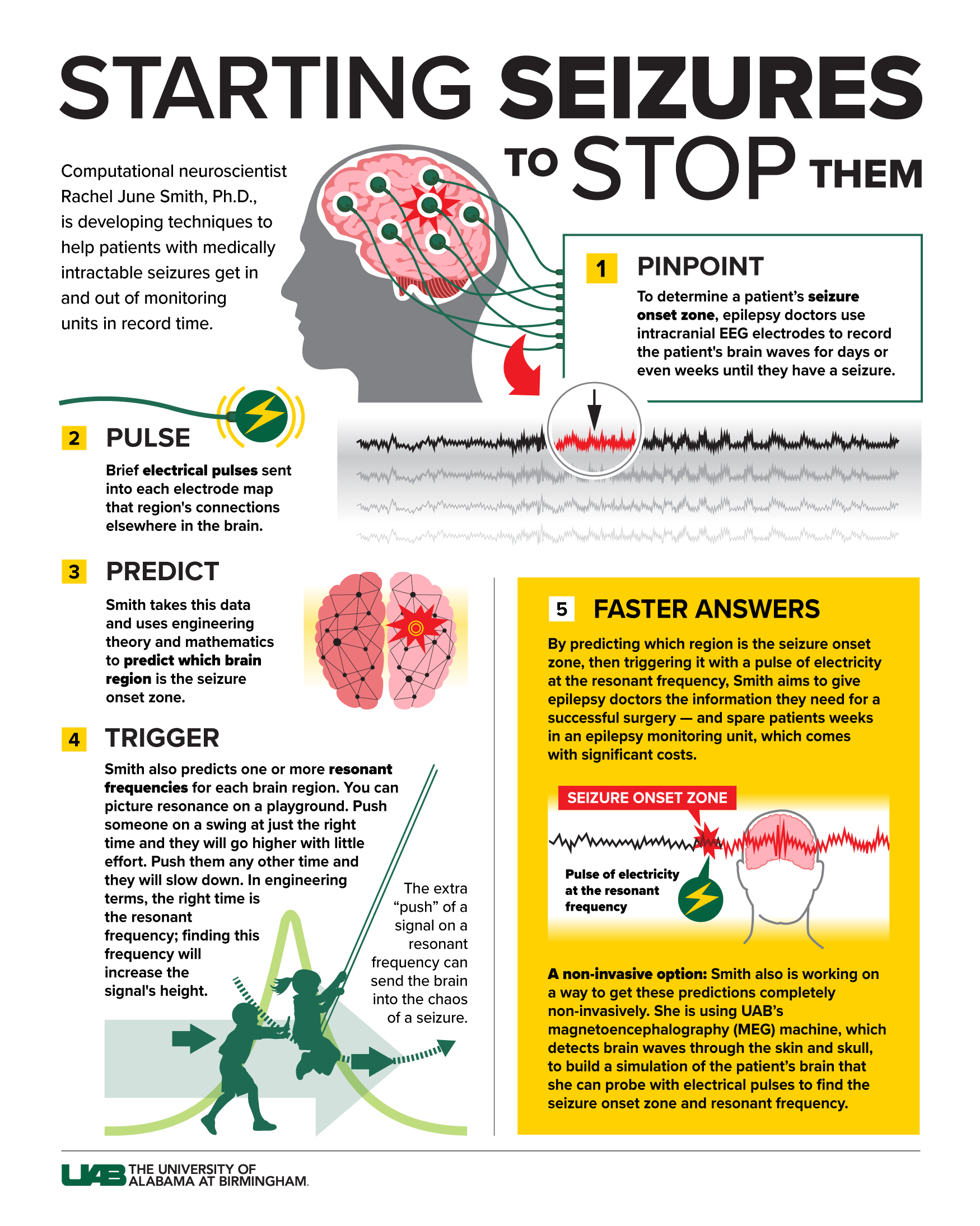 Infographic by JODY POTTER | UAB Marketing and Communications
Infographic by JODY POTTER | UAB Marketing and CommunicationsSeizures, Pokémon and high patient volumes
UAB’s unique combination of high epilepsy patient volume, high-tech equipment, expert epileptologists and the highly interdisciplinary Neuroengineering Initiative convinced Smith to bring the work to Birmingham after her postdoctoral fellowship at Johns Hopkins University. What is she doing, exactly? That’s where Pokémon comes in.
In December 1997, hundreds of Japanese children were admitted to hospitals after watching the cartoon’s 38th episode. The animators had unknowingly made a fateful decision — to depict the episode’s climactic fight, they flashed an explosion onscreen, in alternating red and blue, at 12.5 times per second for about four seconds. Explosions are not uncommon in Pokémon, but the precise frequency — 12 Hertz — triggered the children’s visual networks and threw them into a frenzy.
The children turned out to have photosensitive epileptic events, which are relatively rare. (Photosensitive epilepsy affects anywhere from 3 percent to 5 percent of the 2.7 million Americans with epilepsy, according to the Epilepsy Foundation.) But it is a dramatic example of an engineering phenomenon called resonance. The specific timing of a signal — or frequency, measured in Hertz — can have dramatic effects. “Flashing lights can activate the occipital region of the brain where vision is processed,” Smith said. That led to the question behind the research she is pursuing: “If there is a specific 12 Hertz frequency that activates the occipital lobe, could there be other resonant frequencies for attentional circuits or motor circuits or the circuits that are driving the start of the patient’s seizures?”
 Smith and Brinyark examine data in the Neural Signal Processing and Modeling Lab.Photo by JENNIFER ALSABROOK-TURNER | UAB Marketing and Communications
Smith and Brinyark examine data in the Neural Signal Processing and Modeling Lab.Photo by JENNIFER ALSABROOK-TURNER | UAB Marketing and CommunicationsWhy resonant frequencies are key
Engineers know a lot about the amplifying effects of resonance, which can cause everything from the Tacoma Bridge Collapse to loudspeaker feedback, to the lightning-like sparks of a Tesla coil. Smith likes to use an even more familiar illustration: playground swings. Push a child on a swing at just the right time (that is, the “resonant frequency”), and you can make them go higher and higher with little additional effort. But give them a push just a little too early or late, and they will end up slowing down instead. The same thing can happen in electrical circuits, including the neural circuits that power our brains. Although much of the fundamental causes of epilepsy are still mysterious, researchers hypothesize that pathological, seizure-causing brain tissue is fundamentally unstable. The extra “push” of a signal on a resonant frequency, Smith explains, can send it into the chaos of a seizure.
When a patient is being evaluated with intracranial EEG in an epilepsy monitoring unit, epileptologists are looking for signs of abnormal activity in each of the tens to hundreds of channels on the electrodes inserted into their brains. Primarily they are looking for the characteristic spikes or high-frequency patterns that mark the start of the seizure. But the epileptologists also will map brain networks with single pulses of electrical stimulation. The goal here is to learn, after a brief jolt of electricity, where else do they detect neurons firing on the EEG? They are doing this to know how important brain regions are connected together.
Smith and colleagues at Johns Hopkins found that they can use this data, plugged into a mass of matrix algebra known as a transfer function model, to predict which electrode is actually placed in the seizure onset zone. As they wrote in a 2020 paper, the seizure onset zone should be the one that “generates the ‘largest’ network response to the ‘smallest’ pulse input or ‘kick.’”
But what if you could use those same electrical pulses not to map networks but to start a seizure on command, by finding the resonant frequency and then sending signals back into the seizure onset zone at that frequency? That could give doctors more confidence that they have found the seizure onset zone and save patients time, money and anxiety by getting them out of the seizure monitoring unit much faster.
Smith also has early data indicating that she can possibly localize seizure onset zones without intracranial EEG at all.
Study success leads to funding
In a 2022 paper in the journal Brain, Smith and her collaborators at Johns Hopkins reported that they were able to correctly predict which patients would have a successful surgery with 79 percent accuracy — a major improvement over the 50 percent success rate clinicians have now. They also were able to trigger seizures or auras in six patients by transmitting pulses into their brains at the resonant frequency that they had calculated. Smith’s expansion of this work at UAB is funded by a $50,000 Junior Investigator Award from the American Epilepsy Society.
“We approach patients who are going to undergo intracranial EEG and single-pulse electrical stimulation and ask if they would like to participate in our research,” Smith said. “Most of the time, they say yes. They want to help other people who are going through this.”
Smith also has early data indicating that she can possibly localize seizure onset zones without intracranial EEG at all. This completely non-invasive approach relies on magnetoencephalography, or MEG, which uses magnetic fields to analyze brain patterns. The MEG data allows her to build simulations of the patient’s brain and then stimulate them virtually to predict where the seizure onset zone is located. This work is funded by a $100,000 grant from the nonprofit CURE Epilepsy. “MEG is the perfect modality,” Smith said. “It has high temporal resolution like EEG and high spatial resolution as well. And UAB is one of the few facilities that has a MEG system.”
Advancing this research requires a very specific recipe of requirements that Smith says she did not find anywhere else when looking for her first faculty position.
The main goals of Smith’s research are, “one, can we expedite the process of seizure monitoring, so that we can stimulate and know in one to three days where the seizure onset zone is?” she said. “That opens up the door for a lot more patients to be seen. And, two, can we increase the accuracy, to localize where the seizures are starting and get to an 80 or 90 percent success rate? A lot of people choose not to do surgery because of the low success rate currently.”
Advancing this research requires a very specific recipe of requirements that Smith says she did not find anywhere else when looking for her first faculty position. “The access to MEG is a huge deal,” she said. “For my work, I also have to have a Level 4 epilepsy center — the ones that do these epilepsy surgeries and intracranial monitoring — and there is often only one or two in major U.S. cities. Plus, UAB is such a huge hospital, with some of the highest volume of epilepsy patients anywhere. I interviewed at an institution that had a patient volume about half of what it is here. That means it would take me double the amount of time to do this research.”
Neuroengineering and Brain-Computer Interface Initiative led to Smith's recruitment
UAB’s Neuroengineering and Brain-Computer Interface Initiative, which led to Smith’s recruitment, helps support her research as well. The related doctoral program in Neuroengineering, one of only a few nationwide, has already brought an advanced student into her Neural Signal Processing and Modeling Lab. And the university-wide Consortium for Neuroengineering and Brain-Computer Interfaces has funded some of this work with a $10,000 Pilot Award and helped her build a growing list of collaborators. These include Benjamin Cox, M.D., an assistant professor in the Department of Neurology who regularly performs single-pulse electrical stimulation and uses these data to improve source localization algorithms, and Ismail Mohamed, M.D., a professor in the Division of Pediatric Neurology, who also has research interests in predictors of outcomes after epilepsy surgery and a clinical interest in magnetoencephalography. For his research, Mohamed had already collected MEG data from patients who also had intracranial monitoring, Smith said, which is allowing her “to cross-correlate a resting-state model” for virtual stimulations.
Other collaborators include Arie Nakhmani, Ph.D., professor in the School of Engineering and co-director of the Neural Signal Processing and Modeling Lab, who is an expert in control theory and dynamical systems theory, and Lawrence Ver Hoef, M.D., an associate professor in neurology and an expert epileptologist. Smith also is working with another recent UAB recruit, Adam Goodman, Ph.D., a cognitive neuroscientist in the Department of Psychology who is studying biomarkers for seizures.
“I couldn’t have asked for a better environment,” Smith said.