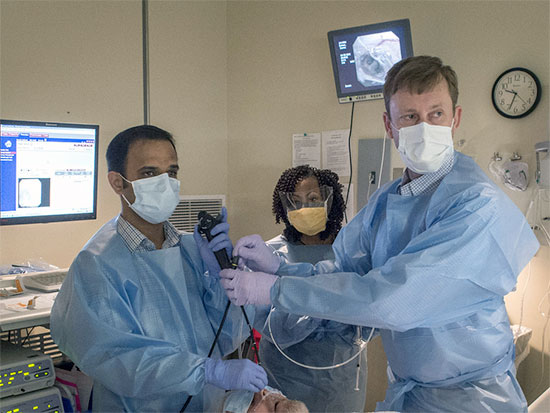
 Surya Bhatt, left, and Mark Dransfield, right, maneuver the valve into place with a bronchoscope.A new approach to treating emphysema has demonstrated significant positive results, according to new findings from a multi-site study. The University of Alabama at Birmingham School of Medicine was one of 24 centers worldwide that studied the new approach, Zephyr® endobronchial valves. Results from the LIBERATE study were published this week in the American Journal of Critical Care Medicine.
Surya Bhatt, left, and Mark Dransfield, right, maneuver the valve into place with a bronchoscope.A new approach to treating emphysema has demonstrated significant positive results, according to new findings from a multi-site study. The University of Alabama at Birmingham School of Medicine was one of 24 centers worldwide that studied the new approach, Zephyr® endobronchial valves. Results from the LIBERATE study were published this week in the American Journal of Critical Care Medicine.
Emphysema is the most severe form of chronic obstructive pulmonary disease, or COPD, which is the third leading cause of death in the United States. There are 11 million Americans diagnosed with the disease. COPD is progressive, and over time it may evolve into emphysema, where patients struggle to breathe.
Zephyr® endobronchial valves are one-way valves the size of a black-eyed pea, 4 to 5.5 millimeters in size. In a minimally invasive procedure using a bronchoscope, the valves are inserted in the lungs to block airflow to diseased regions.
Emphysema, a lung disease usually caused by smoking, damages air sacs in the lung. The sacs fill with air that the body is unable to exhale, causing the lungs to expand. This in turn flattens the diaphragm, the primary muscle used for breathing. The flattened diaphragm is unable to function properly, making it extremely difficult for the individual to breathe.
“The idea behind all lung-volume-reduction procedures is to reduce the volume in the lung and allow the diaphragm to return to its normal shape and function,” said Mark Dransfield, M.D., professor in the Division of Pulmonary, Allergy and Critical Care Medicine, Department of Medicine, and lead UAB investigator for the LIBERATE study. “The Zephyr® endobronchial valves appear to be a less invasive way to achieve that goal without the risks inherent in surgery.”
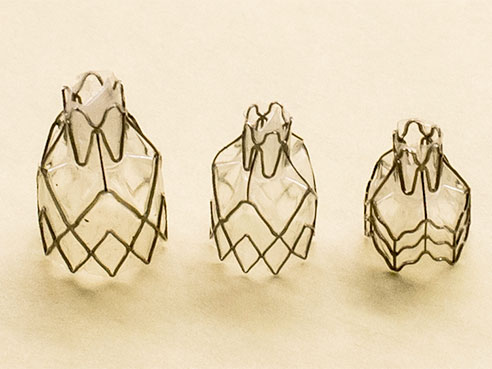 The experimental one-valves used in a study to seal off diseased part of the lungs in patients with severe emphysema.The study followed 190 patients over one year. Results showed that Zephyr valve treatment helped severe emphysema patients breathe better, be more active and enjoy a better quality of life. In the study, patients treated with the Zephyr valve were able to do more daily activities, such as walking, bathing and doing chores, with less shortness of breath than patients treated with medication alone.
The experimental one-valves used in a study to seal off diseased part of the lungs in patients with severe emphysema.The study followed 190 patients over one year. Results showed that Zephyr valve treatment helped severe emphysema patients breathe better, be more active and enjoy a better quality of life. In the study, patients treated with the Zephyr valve were able to do more daily activities, such as walking, bathing and doing chores, with less shortness of breath than patients treated with medication alone.
“Emphysema patients have a terrible quality of life, and this study showed that the Zephyr valve treatment can significantly improve breathing and exercise capacity for these patients,” Dransfield said.
Several different measures of breathing and quality of life were studied, and patients treated with Zephyr valves showed significant improvement across all of them.
In addition, Zephyr patients had a significantly lower rate of respiratory failure and a trend toward a reduction in COPD hospitalization over the course of one year when compared to patients taking only medication.
The most common risk of the procedure was an air leak, known as a pneumothorax, which affected approximately one-third of patients. Most instances were handled through standard medical management.
The Zephyr endobronchial valve is not yet cleared by the FDA or commercially available in the United States. For more information about the Zephyr endobronchial valve, visit www.pulmonx.com or call the UAB Lung Health Center at 205-934-5555.