 Ludwine Messiaen
Ludwine Messiaen
It is easy to tell a medical research story that has a simple and dramatic moment. But disease is often much more complex, and the work to understand it can be painstaking.
A vivid example of that is seen in the UAB Medical Genomics Laboratory, headed by Ludwine Messiaen, Ph.D., professor of genetics. This lab offers clinical genetic testing for a broad array of common and rare genetic disorders. One of the most confounding is neurofibromatosis type 1.
This can be a heartbreaking disease.
Changes at puberty
It usually starts with café-au-lait skin markings, so named because of their distinctive coloring, in an infant. But at puberty — already a challenging time in a person’s life, many patients develop benign skin tumors called neurofibromas that erupt as bumps across the body. Patients vary widely in their symptoms, which can include freckles near skin folds of the body, nodules in the eyes, tumors along the optic nerve, heart defects, anomalies of connective tissue or bones, developmental delay, intellectual disability, and learning problems.
Patients show a broad clinical variability as they grow, and whether their case will be mild or severe cannot — in most cases — be predicted when the disease first appears. This leaves physicians and families uncertain about what symptoms will appear in a particular child as he or she nears puberty.
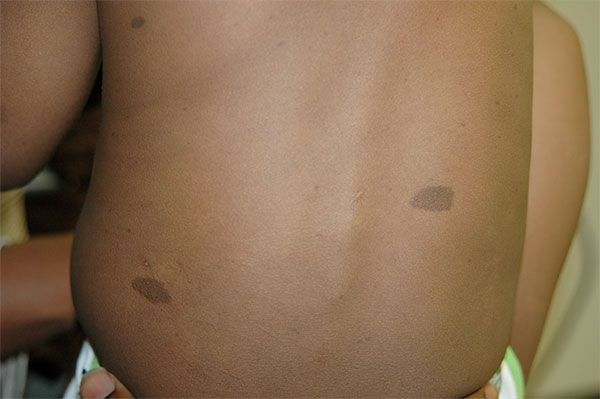 Cafe-au-lait skin markings on the back of a young child
Cafe-au-lait skin markings on the back of a young child
Profusion of mutations
This kaleidoscope of clinical signs is mirrored by an abundance of different mutations in the NF1 gene, responsible for the disease. The UAB Medical Genomics Laboratory has collected DNA and identified a pathogenic mutation in more than 7,800 unrelated neurofibromatosis type 1 patients. All have NF1 mutations, but meticulous examination has revealed so far more than 3,000 different mutations. These can be found in every part of the gene, and the mutational spectrum involves microdeletions, deletions or duplications that involve one or more exons, frameshift and nonsense mutations, and splice or missense mutations. Almost half of the NF1 patients carry a unique mutation found only in their specific family. Other mutations have been found in multiple unrelated families.
Two searches
From this complicated array of mutations and clinical symptoms, Messiaen and her colleagues have tried to answer two questions.
First, can a particular mutation be correlated with the symptoms that will develop as the child grows? This is called a genotype/phenotype (DNA/symptoms) correlation, and only two have previously been found for neurofibromatosis type 1.
“It’s important for people to know what may happen,” Messiaen said. “When a child is born with neurofibromatosis type 1, café-au-lait spots appear very shortly after birth; but other problems, more specifically the development of skin neurofibromas, typically appear around puberty. If a genotype/phenotype correlation exists for a particular mutation, it will help these families have some perspective of what the future will bring, and it will help families cope with the disease. If it is a mutation that takes away the heavy tumor burden at puberty, that information will relieve families, even though learning disabilities may still appear.”
The second question for Messiaen and UAB postdoctoral trainee Meng-Chang “Jack” Hsiao, UAB Department of Genetics, is whether they could identify the likely mechanism that caused a group of mutations in which the DNA has been rearranged to create mix-ups that make the gene longer or shorter.
Each question requires meticulous research. One means reaching out to patients, families and referring physicians around the nation and the world. The other is a molecular genetic detective story, pursued in the UAB lab.
 Messiaen and Meng-Chang "Jack" Hsiao are exploring the mechanisms behind the mutations seen in neurofibromatosis type 1.
Messiaen and Meng-Chang "Jack" Hsiao are exploring the mechanisms behind the mutations seen in neurofibromatosis type 1.
Seeking a correlation
For the first question, Messiaen last year led a group of 74 researchers and clinicians from 58 centers in the discovery of just the third genotype/phenotype correlation ever found for neurofibromatosis type 1. They looked at 136 individuals who all had a missense mutation in the arginine moiety of neurofibromin, the protein encoded by the NF1 gene, at amino acid position 1,809. These mutations are the second-most-frequent ones seen in the UAB collection.
To look for a correlation, the team had to gather detailed clinical symptomatic information for each of the neurofibromatosis patients, from patients, families, referral physicians and researchers in 24 U.S. states and Australia, Belgium, Brazil, Chile, the United Kingdom, India, Israel and Spain.
In a paper published in the journal Human Mutation last year, they found that these patients have a distinct phenotype, Messiaen says. They had the café-au-lait marks, with or without the skin-fold freckling and Lisch eye nodules. But the patients did not develop the visible, disfiguring neurofibromas on their skin around puberty. However, there was a higher prevalence of blood flow obstruction from the heart to the lungs and a short stature. More than half had developmental delays and/or learning disabilities.
Messiaen is calling for international collaboration to expand the study to a total of 250 mutations, which will provide the statistical power needed for patient case management by doctors. And in the next few years, she will focus on finding more genotype/phenotype correlations for other specific mutations.
"If a genotype/phenotype correlation exists for a particular mutation, it will help these families have some perspective of what the future will bring, and it will help families cope with the disease."
| "If a genotype/phenotype correlation exists for a particular mutation, it will help these families have some perspective of what the future will bring, and it will help families cope with the disease." |
Chasing molecular clues
For the second question, Hsiao, Messiaen and colleagues looked at NF1 copy-number variations — where the mutant gene is either longer or shorter than a normal NF1 gene — from 85 unrelated neurofibromatosis type 1 patients, along with two previously published copy-number variations. Ten of these were partial duplications within the NF1 gene, and 77 were deletions. Hsiao looked for specific nucleotide breakpoints in these variants — the places where the duplication or deletion begins or ends — that would be clues to how the changes occurred.
The methods to examine these mutant genes include multiplex ligation-dependent probe amplification, array comparative genomic hybridization, breakpoint-spanning PCR and sequencing.
“The most difficult challenge is to see how the rearrangements happen,” Hsiao said. “It’s really difficult to decipher.”
In a paper published in The American Journal of Human Genetics last year, Hsiao found that DNA replication-based mechanisms — such as fork stalling and template switching, and microhomology-mediated break-induced replication — as well as serial replication stalling appear to be the major causes of the NF1 copy-number variants. In one complicated rearrangement, the DNA replication appeared to have stalled five times, with the stalled DNA strand then either invading forward or invading backward into another part of the NF1 gene. Hsiao also found that the mutant genes showed rearrangement hotspots that included one palindromic sequence and four Alu elements. Alu elements are short primate-specific repeats in the DNA; the human genome contains about 1 million copies of various Alu elements that make up almost 11 percent of the genome.
Two sides to the research
Messiaen says the two recent papers are “nice companions.”
“They show two sides of research aspects of this laboratory,” she said. “One digs deeper into the mechanism of specific types of mutation, and one contributes to genotype/phenotype correlation.”