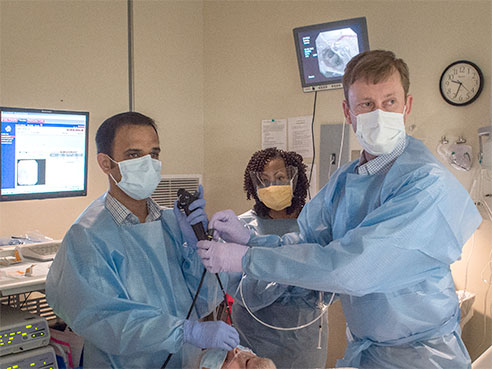
 Investigators at the University of Alabama at Birmingham are investigating whether a one-way valve placed in the lungs of patients with severe emphysema will help improve lung function. It is one of several techniques being developed to reduce lung volume without surgery.
Investigators at the University of Alabama at Birmingham are investigating whether a one-way valve placed in the lungs of patients with severe emphysema will help improve lung function. It is one of several techniques being developed to reduce lung volume without surgery.About 4.9 million Americans have been diagnosed with emphysema, a lung disease usually caused by smoking. It damages air sacs in the lung called alveoli. The sacs fill with air that the body is unable to exhale, causing the lungs to expand. This in turn flattens the diaphragm, the primary muscle used for breathing. The flattened diaphragm is unable to function properly, making it extremely difficult for the individual to breathe.
One longstanding therapy is lung-volume-reduction surgery, in which over-inflated, diseased parts of the lung are surgically cut away, allowing the lung to return to a more normal size. This allows the diaphragm to return to normal function. Surgery is effective, but commonly not done in the United States due to perceived risks for pulmonary or cardiac complications.
The current UAB trial is called the LIBERATE Study, using the experimental Zephyr® endobronchial valve. In a minimally invasive procedure using a bronchoscope inserted in the airway, several small, one-way valves are placed in the lungs to block airflow to diseased regions. This allows healthy regions to expand and function more efficiently, enabling better breathing and improving quality of life.
“The idea behind all lung-volume-reduction procedures is to reduce the volume in the lung and allow the diaphragm to return to its normal shape and function,” said Mark Dransfield, M.D., associate professor in the Division of Pulmonary, Allergy and Critical Care Medicine and medical director of the UAB Lung Health Center. “We’re looking for a less invasive way to achieve that goal without the risks inherent in surgery.”
Dransfield has implanted the valve in four patients with severe emphysema. Typically, a patient gets four or five valves in one side of a lung. Not all patients with severe emphysema are candidates for the study.
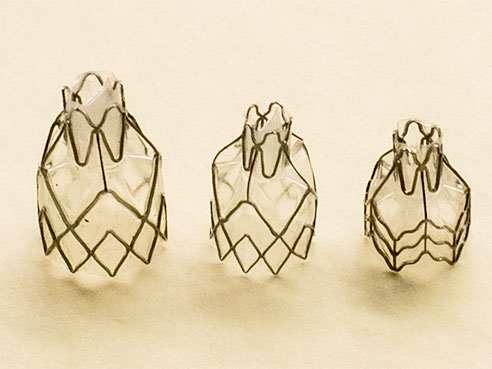 The experimental one-valves used in a study to seal off diseased part of the lungs in patients with severe emphysema.“Patients need to have enough healthy lung tissue so that the blockage of the most diseased and damaged areas and the reduced lung volume will allow the healthier areas to function more normally,” Dransfield said.
The experimental one-valves used in a study to seal off diseased part of the lungs in patients with severe emphysema.“Patients need to have enough healthy lung tissue so that the blockage of the most diseased and damaged areas and the reduced lung volume will allow the healthier areas to function more normally,” Dransfield said.The procedure is not a cure for emphysema; but Dransfield says clinical studies in Europe have indicated the majority of patients see a significant improvement in lung function, exercise tolerance and quality of life. More than 25,000 Zephyr valves have been implanted outside the United States in the past 10 years.
Results from the LIBERATE Study will be used as part of the submission seeking approval from the FDA.
For more information about the LIBERATE clinical trial, call the UAB Lung Health Center at 205-934-5555 or visit www.pulmonx.com or call 888-248-5864.