 by Hannah Buckelew
by Hannah Buckelew
Zdenek Hel, Ph.D., a professor in the UAB Department of Pathology’s Division of Molecular and Cellular Pathology, received a five-year, $3.6 million R01 to support his project, “Dysregulated neutrophil subpopulations as a driving mechanism of liver and gastrointestinal disease in HIV-1-infected individuals.” The grant is funded by the National Institute of Diabetes and Digestive and Kidney Diseases and will run from July 2023 through May 2028.
Patients infected with HIV-1 often experience gastrointestinal (GI) mucosal damage and destruction of the gut’s epithelial barrier. Breaches of this barrier result in an increased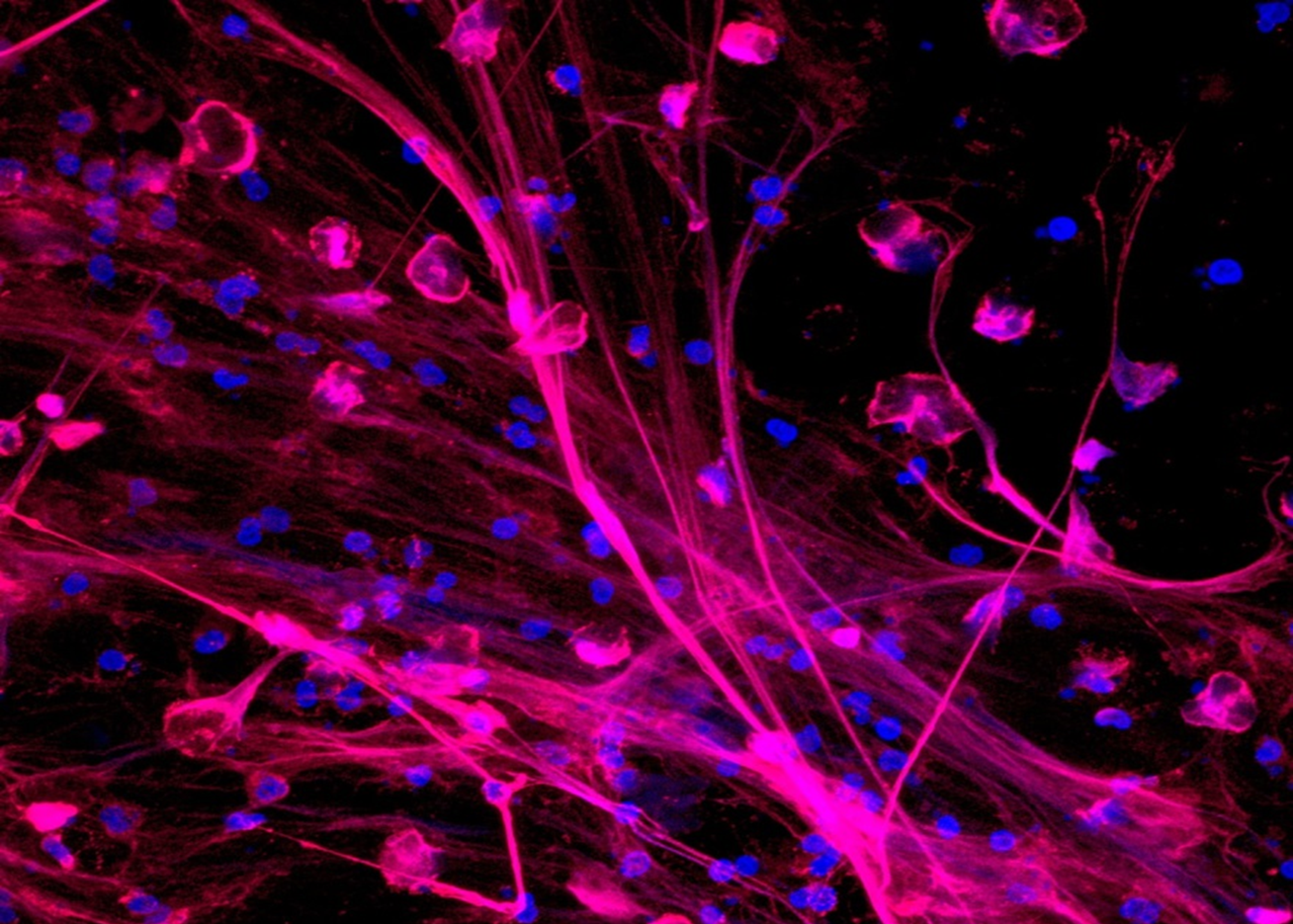 Neutrophils undergoing NETosis intestinal permeability, allowing unwanted bacteria and microbial products to enter through. These breaches can cause chronic immune activation and inflammation and, as a result, an increase in mortality in people living with the HIV-1 infection.
Neutrophils undergoing NETosis intestinal permeability, allowing unwanted bacteria and microbial products to enter through. These breaches can cause chronic immune activation and inflammation and, as a result, an increase in mortality in people living with the HIV-1 infection.
Mounting evidence suggests that neutrophils play a critical role in GI and liver damage in HIV-1. Neutrophils are a type of white blood cell that act as the body’s first line of defense against invading microbial and viral pathogens and represent the most abundant immune cell population.
“The liver serves as a firewall for capturing bacteria and bacterial products in the blood,” says Hel. “We found that circulating neutrophils in individuals with HIV-1 became highly activated and released products that promote damage in the gut and liver to drive the progression of GI and liver disease.”
Hel’s previous research found that circulating neutrophils from patients with HIV-1 display properties indicating that they are highly activated and undergo NETosis, a process in which large extracellular “nets” are produced. NETosis is a program for the formation of neutrophil extracellular traps, or NETs, composed of chromosomal DNA and lytic enzymes. NETs and other neutrophil products induce epithelial and endothelial damage, provide the stimulus and the scaffold for thrombus formation, and prime macrophages to produce cytokines that amplify immune cell recruitment to local inflammatory lesions.
HIV-1 infection induces neutrophil populations with a high capacity to produce reactive oxygen species and undergo NETosis. The products released from activated neutrophils promote damage in the gut mucosa and liver and drive the progression of GI and liver disease.
The overall objectives of this study are to define the role of neutrophil subpopulations and NETosis as driving mechanisms of GI and liver damage, and to identify the mechanisms responsible for chronic neutrophilic activation in HIV-1 to reveal the specific checkpoints for intervention. Hel’s research team will accomplish these by implementing a novel sequencing method (CITE-Seq) based on cellular indexing of transcriptomes and epitopes.
Hel’s primary collaborator on this study is Paul Goepfert, M.D., Professor in the UAB Department of Medicine’s Division of Infectious Diseases and director of the Alabama Vaccine Research Clinic.
“Liver disease has become the second-most common cause of death in people living with HIV-1,” says Hel. “Neutrophils can be pharmaceutically targeted, so we’re hopeful this research will have an important translational impact and open new avenues for innovative treatment approaches.”