 The endoplasmic reticulum (ER) regulates protein folding and maintains proteostasis in cells- a process essential to maintaining cellular health. However, researchers at UAB have found that chronic reductive stress (RS) impairs the ER transcriptome in the hearts of mice. In fact, RS has been found in the pathophysiology of several diseases, notably cancer, neurodegeneration and metabolic disorders.
The endoplasmic reticulum (ER) regulates protein folding and maintains proteostasis in cells- a process essential to maintaining cellular health. However, researchers at UAB have found that chronic reductive stress (RS) impairs the ER transcriptome in the hearts of mice. In fact, RS has been found in the pathophysiology of several diseases, notably cancer, neurodegeneration and metabolic disorders.
In a recent study published in Redox Biology, Rajasekaran Namakkal-Soorappan, Ph.D., an associate professor in the Division of Molecular and Cellular Pathology, found that prolonged moderate treadmill exercise mitigates the RS-induced ER dysfunction and cardiac remodeling in mice.
“Our findings underscore the potential of exercise in mitigating RS-associated pathology, highlighting its essential role in maintaining cellular proteostasis,” says Namakkal-Soorappan.
Using RNA sequencing, findings showed notable alterations in the ER transcriptome of the hearts at 4, 12 and 24 weeks. The downregulation of ER genes was notably significant at 12 weeks, and further pronounced at 24 weeks. Specifically, exercise intervention for 20 weeks, beginning at 6 weeks of age, reduced cardiomyocyte hypertrophy (thickening of the heart muscle) in mice through adaptive remodeling, and preserved the cardiac function.
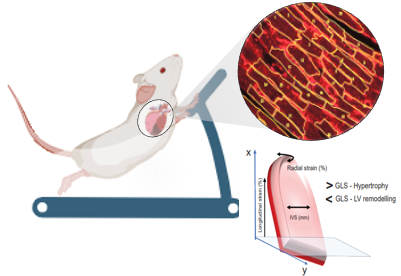 Namakkal-Soorappan and his team found that exercise preserves the systolic and diastolic functions in RS hearts, partially rescues ER proteins and activates autophagy signaling in mice, augments adaptive remodeling and prevents pathological remodeling in the cardiomyocytes of hearts.
Namakkal-Soorappan and his team found that exercise preserves the systolic and diastolic functions in RS hearts, partially rescues ER proteins and activates autophagy signaling in mice, augments adaptive remodeling and prevents pathological remodeling in the cardiomyocytes of hearts.
Silvio Litovsky, M.D., Professor, Division of Anatomic Pathology and a key collaborator in this study, highlighted the adaptive remodeling of the cardiac cells growing longer (physiological) instead of getting wider (pathological) during the treadmill exercise, which is a key finding to translate in humans with cardiac hypertrophy. Through exercise, one can increase the length of the cardiomyocyte while preventing any genetic/life-style driven cellular hypertrophy (reducing the length and increasing the width), which compromises the cells' ability to contract and efficiently pump as a whole heart. 
Namakkal-Soorappan acknowledged the unique improvement in global longitudinal strain following exercise. He also noted an increase in cardiac output in the exercised mice compared to control mice, while the ejection fraction remained comparable between the two groups.
“Exercise can trigger physiological adaptations despite an unchanged antioxidative burden in the heart,” he added.
Dr. Sini Sunny, a recipient of an AHA Postdoctoral Fellowship working in Dr. Namakkal-Soorappan’s lab, highlighted the positive impact of exercise on cardiac function in transgenic mice experiencing antioxidative stress.
Namakkal-Soorappan’s collaborators on this study include Vivek Nanda, Silvio Litovsky, UAB Department of Pathology, Arun Jyothidasan, Aniqa Sayed, Sini Sunny, John Kofi Afortude, UAB Cardiac Aging and Redox Signaling Laboratory, Steven Pogwizd, UAB Department of Medicine, Matthew Might, UAB Hugh Kaul Precision Medicine Institute, Brian Dalley and Trinity Joel, University of Utah School of Medicine.